Washington University SPORE in Pancreatic Cancer
Washington University
Principal Investigator(s):

David DeNardo, PhD

William Hawkins, MD

Ryan Fields, MD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Employing CD11b-Agonists to Render PDAC Responsive to Immunotherapy
- Project 2: Mechanisms of Resistance to Neoantigen Vaccines to PDAC
- Project 3: Targeting Stress-Induced MK2 as Novel Strategy in Pancreatic Cancer
- Administrative Core
- Biospecimen Core
- Biostatistics Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigator(s) Contact Information
David DeNardo, PhD
Professor of Medicine and Pathology/Immunology
Co-Director Tumor-Immunology Program, Siteman Cancer Center
Washington University School of Medicine MSC 8069-0004-07
Washington University
660 South Euclid Avenue
St. Louis, MO 63110
(314) 747-2797
William Hawkins, MD
Deputy Director, MUSC Hollings Cancer Center
Medical University of South Carolina Hollings Cancer Center
86 Jonathan Lucas Street| HO321S
Charleston, SC 29425
(843)-974-2066
Ryan Fields, MD
Chief, Surgical Oncology
Director, Washington University Solid Tumor Tissue Bank and Registry
Washington University
660 South Euclid Avenue
St. Louis, MO 63110
(314) 286-1694
Overview
Washington University’s Pancreas SPORE is designed to address the deadliest form of pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC), by collaborating with multiple departments, programs, and other institutions in interdisciplinary translational research. The Pancreas SPORE investigators have expertise in basic and clinical sciences, and individual expertise in immunology, drug development, genomics, and imaging to develop novel therapeutic approaches to PDAC.
Our translational research program possesses both breadth and depth. Our team has repeatedly demonstrated its ability to translate basic science discoveries to therapeutic approaches. Recent examples of this ability include advances in our understanding of the tumor micro-environment (TME) or tumor-mediated immune suppression, which have successfully moved from discovery to preclinical models and ultimately into clinical trials. Our group has also successfully moved findings from the clinic back to the bench. For example, the rebound observed in other myeloid subsets when using CCR2 inhibitors led to testing of CCR2/5 inhibition in subsequent research efforts. The Washington University SPORE in Pancreatic Cancer at Siteman Cancer Center includes patients with all disease stages, and its trials are led by many investigators from multiple disciplines. The clinical program is well established, and our growing reputation has facilitated academic and clinical partnerships, leading to numerous clinical trial opportunities. Over the last four years, pancreatic cancer patients have been enrolled in 103 studies, 41 of which are therapeutic clinical trials at Siteman.
The Pancreas SPORE includes three research programs, an administrative core and two shared resource cores, and research opportunities for collaboration, including developmental research and career enhancement programs. Clinical trials are an important and active part of the Pancreas SPORE. The long-term goal of the Pancreas SPORE is to improve PDAC patient survival. To achieve this goal, our SPORE will collaborate both within Washington University and with external institutions. Our investigators expect no singular approach to solve PDAC and fully commit to supporting young investigators and evaluating new ideas. Our SPORE will provide access to pancreas cancer-specific resources to further this goal.
Project 1: Employing CD11b-Agonists to Render PDAC Responsive to Immunotherapy
Project Co-Leaders:
David G. DeNardo, PhD (Basic)
Katrina Pedersen, MD (Clinical)
Project 1 of the Pancreas SPORE seeks to understand if targeting integrins on myeloid cells can impact therapeutic responsiveness to immunotherapy in pancreas cancer. They include studies clinically testing an integrin agonist in PDAC patients and correlative and laboratory studies to understand mechanism of resistance.
Research Overview of Project 1:
The potential of checkpoint immunotherapy to combat cancer has been established in several cancer types. However, in pancreatic ductal adenocarcinoma (PDAC), checkpoint immunotherapy has not yet led to clinical benefits. Although multiple factors likely contribute to checkpoint resistance, one significant factor is extensive infiltration of PDACs by multiple lineages of immunosuppressive myeloid cells. These myeloid cells, which include tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), drive T cell exclusion and dysfunction. Thus, one promising therapeutic strategy is to reprogram these myeloid cells to improve T cell-mediated immunity.
Our team has recently developed an allosteric AGONIST of CD11b, A-008. Our published data demonstrate that CD11b-agonism rapidly repolarizes TAMs to support anti-tumor immunity and combining CD11b-agonists with checkpoint immunotherapy leads to tumor regression and long-term survival in pre-clinical PDAC models. These strong data drive our hypothesis that CD11b agonism reprograms the PDAC tumor microenvironment to overcome resistance to checkpoint immunotherapy. To test this hypothesis, we will:
Aim 1: Determine the safety and efficacy of A-008, Gemcitabine and Abraxane and PD-1 blockade in metastatic PDAC patients.
Aim 2: Determine the biomarkers of exposure, response, and resistance to A-008 therapy in PDAC patients.
Aim 3: Determine how tumor-intrinsic and extrinsic factors regulate the impact of CD11b agonist therapy. Impact: These studies will investigate a novel approach to render PDAC responsive to immunotherapy.
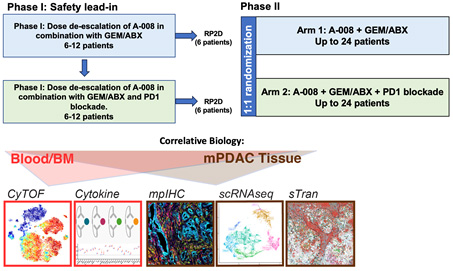
FIGURE: Trial Schematic
Project 2: Mechanisms of Resistance to Neoantigen Vaccines to PDAC
Project Co-Leaders:
William Hawkins, MD (Clinical)
William Gillanders, MD (Basic)
Robert Schrieber, PhD (Basic)
Project 2 of the Pancreas SPORE at Washington University plans to use a novel class of optimized neoantigen synthetic long peptide (SLP) vaccines to induce immune responses against class II antigens in pancreatic cancer. Cancer neoantigens have been identified by us and others as important targets of cancer immunotherapy, including immune checkpoint inhibitors (ICI), adoptive cell therapy, and vaccine therapy. Our initial clinical experience targeting neoantigens in pancreatic cancer confirmed that neoantigen DNA and synthetic long peptide vaccines are capable of generating neoantigen-specific T cell responses in patients. With support from our previous SPORE in Pancreatic Cancer and Stand Up to Cancer, we have been testing the safety and immunogenicity of pancreatic cancer neoantigen DNA vaccines (NCT03122106) and synthetic long peptide vaccines (NCT03956056) following surgery and adjuvant therapy. These clinical trials are providing insights into the most effective neoantigen vaccine platform (DNA vs. synthetic long peptide), Preliminary analyses confirm that both neoantigen vaccine platforms can induce robust immune responses to PDAC neoantigens and suggest that PDAC patients treated with neoantigen vaccines have better than predicted clinical outcomes.
Research Overview of Project 2:
We have made important contributions to the immunobiology of cancer neoantigens, and have developed a robust, publicly available, and frequently downloaded suite of software tools for neoantigen prediction. We recently developed algorithms for the prioritization of class II neoantigens and demonstrated that optimized vaccines incorporating both class I and II neoantigens improve the success of neoantigen vaccines. With funding from Leidos Biomedical Research, we are currently testing optimized neoantigen SLP vaccines in PDAC patients using a window trial design (NCT05111353).
Specific Aims:
Aim 1: Test the hypothesis that optimized neoantigen vaccines can increase the number and improve the function of neoantigen-specific T cells in PDAC.
Aim 2: Test innovative strategies to address the paucity of cDC1 in PDAC.
Aim 3: Test the hypothesis that the TIGIT pathway restrains the response to optimized neoantigen vaccines in PDAC.
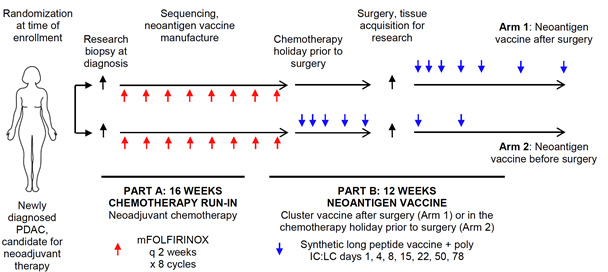
Figure 4. Schematic of phase 1 clinical trial design. Patients with newly diagnosed PDAC who are candidates for neoadjuvant chemotherapy are eligible for enrollment. Subjects will be randomized at the time of enrollment. Subjects will complete 4 months of neoadjuvant chemotherapy (mFOLFIRINOX), and will then be treated with a neoantigen vaccine following surgery (Arm 1), or neoantigen vaccine in the chemotherapy holiday prior to surgery (Arm 2). Subjects in Arm 2 will receive vaccine booster doses following surgery.
Project 3: Targeting Stress-Induced MK2 as Novel Strategy in Pancreatic Cancer.
Project Co-Leaders:
Kian Lim, MD (Clinical)
Gregory Beatty, MD, PhD (Basic)
Project 3 proposes to conduct a phase 1 clinical trial combining a novel MK2 inhibitor ATI-450 with FOLFIRINOX chemotherapy for patients with metastatic pancreatic cancer and to develop a novel immunotherapeutic strategy that can be tested in the future.
Research Overview of Project 3:
To date, combination chemotherapies remain the mainstay treatment of pancreatic cancer. FOLFIRINOX is a combination chemotherapy regimen with the best track record for treatment of pancreatic cancer that cannot be treated with surgery; however, even with the best treatment, the majority of the patients will still succumb to the disease within one to two years of diagnosis. This is largely due to the extreme resistance of pancreatic cancer cells to chemotherapeutics. Using an unbiased protein array analysis, we now made a new discovery that found that pancreas cancer cells dramatically upregulate MK2 enzyme and its partnering molecule Hsp27, when exposed to FOLFIRINOX chemotherapy. Both MK2 and Hsp27 protect pancreas cancer cells from cell death when their DNA is hit by chemotherapy. When MK2 was blocked by a new inhibitor ATI-450, pancreatic cancer cells became much more vulnerable to chemotherapy-induced death. Importantly, ATI-450 is now already in clinical trial for patients with moderate to severe rheumatoid arthritis, has mild side effects and is overall very well tolerated. Therefore, ATI-450 not only has the potential of augmenting the efficacy of chemotherapy but also could mitigate chemotherapy-related side effects.
Specific Aims:
Aim 1. To conduct a phase I study to evaluate the safety and preliminary efficacy of ATI-450 in combination with FOLFIRINOX for patients with locally advanced or metastatic PDAC.
Aim 2. To conduct correlative analyses of tumor and blood samples obtained from trial participants to understand the immunological impact and resistance mechanism of ATI-450 plus FOLFIRINOX.
Aim 3. To develop an effective immunotherapy regimen with ATI-450 in mouse models.
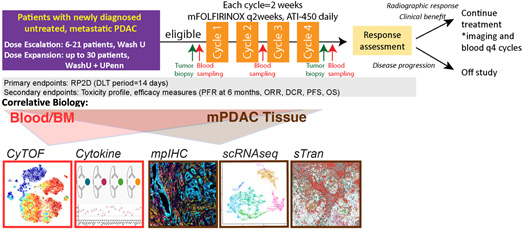
Figure: A) Overall study scheme and B) dosing regimen of the proposed phase 1 clinical trial. In dose escalation phase, 6-21 patients with locally advanced or metastatic PDAC will be enrolled to determine RP2D based on BOIN design. Once R2PD is achieved, up to 30 patients will be enrolled in dose expansion phase. Patients are treated with mFOLFIRINOX every two weeks and ATI-450 BID until disease progression or intolerable toxicities. Tumor biopsies are done before treatment and after 4 cycles (8 weeks), and blood samples will be collected every 2 weeks initially and every 4 weeks afterwards.
Administrative Core
Core Directors:
William Hawkins, MD (Core Lead)
David DeNardo, PhD (Core Lead)
Ryan Fields, MD (Core Lead)
The goal of the Administrative Core is to provide executive oversight and administrative support for all of the Pancreatic Cancer SPORE projects and cores. The Administrative Core also monitors the activities of all program components, ensures compliance with local and federal grant administration guidelines, and facilitates communication and collaboration among program members.
Specific Aims:
Aim 1: Facilitate intra- and inter-SPORE communication and collaboration including development and maintenance of this website to provide real-time progress updates and contact information. In addition, the core plans and executes bi-monthly working group meetings, monthly Steering Committee meetings, and an annual retreat to facilitate the exchange of ideas and the use of shared resources.
Aim 2: Provide administrative and fiscal oversight and support for all SPORE components.
Aim 3: Coordinate the SPORE Developmental Research Program and Career Enhancement Program administrative activities.
Aim 4: Enhances participation of qualified scientists from a broad array of demographic groups in all SPORE activities.
Aim 5: Ensures that advocacy issues are properly addressed and included in all aspects of research with patient participants.
Aim 6: Assists investigators with preparing scholarly presentations, publications, regulatory documents and all other SPORE-related products.
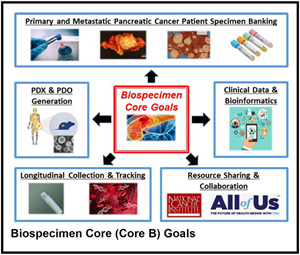
Biospecimen Core
Core Directors:
Ryan Fields, MD (Core Director)
Mark Watson, MD, PhD (Core Associate Director)
The Biospecimen Core is responsible for the identification, enrollment and collection of specimens from adult patients referred to the Siteman Cancer Center (SCC) with newly diagnosed PDAC. The pathologic material from these patients will be banked utilizing our existing collaboration with the SCC Tissue Procurement Core, drawing on caTissue and other informatics resources developed by the Center for Biomedical Informatics. Clinical data will be annotated and prospectively maintained in a robust clinical database. The Biospecimen Core is integrated with and extends the success of our existing Solid Tumor Tissue Bank and Registry.
Specific Aims:
Aim 1: Prospectively identify and bank specimens from patients with PDAC who are referred to the WUSM SCC.
Aim 2: Prospectively maintain a clinical database that captures demographic, epidemiologic, pretreatment, treatment, and follow-up/outcome data from all patients included in the biospecimen collection.
Aim 3: Create patient-derived organoids, xenografts, and cell lines from tumor samples harvested from patients with PDAC at the time of surgery.
Aim 4: Create a longitudinal biobank of tumor biopsies, peripheral blood mononuclear cells, and cell-free DNA from patients with PDAC throughout their treatment course.
Biostatistics Core
Core Directors:
Lu Esther, PhD (Core Director)
Malachi Griffith, PhD (Core Associate Director)
The Biostatistics Core provides pipeline development, statistical design, data management and computational support for all Pancreatic Cancer SPORE investigators and projects. The Biostatistics Core staff will support consultation and collaboration on all aspects of study design, database development and quality control, and analysis, interpretation and presentation of data. The statisticians, epidemiologist and the database manager participating in the Biostatistics Core have a strong record of collaboration, and have been specifically chosen for their broad range of expertise in and experience with clinical trials, laboratory experiments, genetics, and genomics research. Through participation in specific projects and leadership activities, the interrelationships and synergy with the investigator team will accelerate the acquisition of knowledge beyond that which would be expected if these projects were implemented individually, or with research teams that were not interdisciplinary.
Specific Aims:
Aim 1: Provide biostatistical and bioinformatics collaborations for SPORE Projects, and Developmental Research Program studies to ensure that robust statistical methods and reproducible omics analyses are available to support SPORE investigators.
Aim 2: Provide biostatistical and bioinformatics support and training to junior investigators through the Career Enhancement Program.
Developmental Research Program
Program Directors:
Ryan Fields, MD
David DeNardo, PhD
The goal of the SPORE in Pancreatic Cancer Developmental Research Program (DRP) is to recruit and support developmental research projects in pancreatic cancer for future incorporation as full SPORE projects or as the basis for applications for other nationally competitive peer-reviewed funding. The types of pancreatic cancer research projects that will be supported include basic, translational, clinical, epidemiologic, and cancer prevention. Projects supported by the DRP will expand the scope of translational research and increase the number of investigators committed to pancreatic cancer research. The DRP will work in tandem with the Career Enhancement Program (CEP) to assist in developing junior investigators. These objectives will be accomplished by performing four specific aims:
Aim 1: Support the development of pancreatic cancer research projects for future incorporation as full SPORE projects or for new independent peer-reviewed funding.
Aim 2: Foster collaborations between basic and clinical researchers.
Aim 3: Mentor junior faculty and new faculty involved in pancreatic cancer research.
Aim 4: Promote the participation of qualified investigators in pancreatic cancer research and facilitate the recruitment of patients from a wide array of different populations into clinical pancreatic cancer trials.
Career Enhancement Program
Program Directors:
William Gillanders, MD
William Hawkins, MD
The SPORE in Pancreatic Cancer Career Enhancement Program (CEP) aims to recruit and support new independent investigators in translational pancreatic cancer research. Along with the Developmental Research Program (DRP), the CEP will provide financial support, didactic training, and mentored research opportunities to prepare investigators for independent careers in translational pancreatic cancer research. Washington University has a distinguished track record of mentoring successful academic investigators in basic science and translational research. We propose the following specific aims:
Aim 1: Recruit and support new investigators in translational pancreatic cancer research. In addition to the NCI funds, a total of $323,000 in institutional support is committed in support of the CEP program.
Aim 2: Train and mentor junior faculty in translational pancreatic cancer research.
Aim 3: Foster inter-SPORE collaborations.
Aim 4: Promote participation of qualified investigators in pancreatic cancer research.







