Selective Targeting of Pancreatic Cancer (SToP) SPORE
The University of North Carolina at Chapel Hill
Principal Investigator:

Jen Jen Yeh, MD
- Principal Investigator Contact Information
- Overview
- Project 1. Targeting autophagy for the treatment of KRAS-mutant pancreatic cancer
- Project 2. Combined T cell therapy for PDAC
- Project 3. Transcriptomic subtypes, therapy selection and response
- Administrative Core
- Tissue Procurement, Pathology, and Genomics Core
- Integrative Quantitative Sciences Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigators Contact Information
Jen Jen Yeh, MD
Professor & Vice Chair of Research, Surgery, Division Of Oncology
Professor, Pharmacology
Member, Lineberger Comprehensive Cancer Center
University of North Carolina
450 West Drive, CB7295
Chapel Hill, NC 27599
Tel: 919-966-5221
Overview
The main goal of the Selective Targeting of Pancreatic (SToP) Cancer SPORE is to further enhance treatment solutions for pancreatic cancer by focusing on key challenges to identifying, producing, and directing new therapies. The UNC Lineberger Pancreatic Cancer Center of Excellence (PCCE) invests heavily in developing innovative projects, expanding essential infrastructure, and recruiting talented faculty to position UNC as a leader in translational and basic approaches to pancreatic cancer. Thus, the SToP SPORE is prepared to address such challenges and improve the methods we use to treat patients with pancreatic cancer.
Project 1. Targeting autophagy for the treatment of KRAS-mutant pancreatic cancer
Project 2. Combined T cell therapy for PDAC
Project 3. Transcriptomic subtypes, therapy selection and response
Project 1: Targeting autophagy for the treatment of KRAS-mutant pancreatic cancer
Project Co-Leaders:
Channing Der, PhD (Basic Co-Leader)
Ashwin Somasundaram, MD (Clinical Co-Leader)
This project will develop novel combination therapies to target autophagy for the treatment of KRAS-mutant pancreatic ductal adenocarcinoma (PDAC). Results from recent studies of MEK inhibition suggest that ERK inhibitors will have superior activity in PDAC, prompting initiation of a phase II clinical trial with the ERK inhibitor LY3214996 in combination with hydroxychloroquine (HCQ) for PDAC. Although early observations from compassionate care utilization of this combination demonstrate significant clinical impact, preliminary studies support the premise that this therapy can be improved upon. Studies will identify genes that modulate HCQ anti-tumor activity, based on application of a 2,500-gene druggable genome CRISPR-Cas9 genetic-loss-of-function screen. Identified hits that enhance or reduce HCQ growth-inhibitor activity represent candidate combinations or biomarkers for HCQ resistance, respectively. Studies will involve application of a chemical library screen using a 525-oncology drug set to identify combinations that enhance cytotoxicity of HCQ.
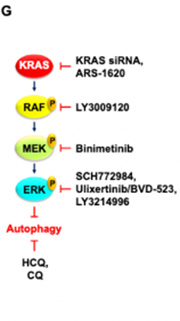
Description of Project 1.
Project 2: Combined T cell therapy for PDAC
Project Co-Leaders:
Gianpietro Dotti, PhD (Basic Co-Leader)
Ashwin Somasundaram, MD (Clinical Co-Leader)
This project will develop new generation adoptive T cell therapies and a B7-H3 selective chimeric antigen receptor (CAR) T cell for pancreatic ductal adenocarcinoma (PDAC). Included is a phase I clinical study in patients with PDAC to assess safety and antitumor activity of B7-H3.CAR-Ts that also contain the inducible caspase9 as a safety switch to terminate the activity of B7-H3.CAR-Ts in case of toxicity. Preclinical models will be developed with CAR-Ts that contain T cells rendered tumor-specific through CAR expression and equipped to overcome the desmoplastic nature of PDAC. Macrophages and myeloid-derived suppressor cells (MDSC) will be reprogramed to a non-immunosuppressive state using TAM receptor tyrosine kinase (RTK) small molecule inhibitors.
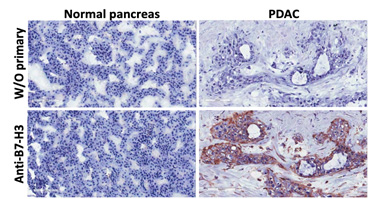
Description of Project 2.
Project 3: Transcriptomic subtypes, therapy selection and response
Project Co-Leaders:
Gary Johnson, PhD (Basic Co-Leader)
Jen Jen Yeh, MD (Clinical/Applied Co-Leader)
This project seeks to alter how treatment selection is directed to FOLFIRINOX and gemcitabine plus nab-paclitaxel (GnP) for metastatic pancreatic ductal adenocarcinoma (PDAC) using novel computational approaches to find alterations in the tumor microenvironment in response to treatment. Studies will evaluate whether a single-sample classifier (PurIST) can help direct treatment selection, and a clinical trial will test PurIST subtyping to direct initial chemotherapy and determine if precision oncology approaches can improve outcomes for patients otherwise less responsive to either FOLFIRINOX or GnP. Studies will also determine whether specific characteristics in the tumor and tumor microenvironment predict response to different therapies. Innovative proteomic approaches will examine the effectiveness of inhibitors in PDAC that might have been overlooked, and might be promising targets.
Administrative Core
Core Directors:
Channing Der, PhD
Hanna Sanoff, MD
Providing a one-stop administration for the SToP Cancer SPORE’s investigators, the goal of the Administrative Core is to aggregate scientific leadership and evaluation; oversee financial matters; conduct planning, scheduling and reporting functions; and administer the Developmental Research Program and Career Enhancement Program. Additionally, the Administrative Core supports investigators and personnel in projects 1-3; the Tissue Procurement, Pathology, and Genomics Core; and the Integrative Quantitative Sciences Core.
Tissue Procurement, Pathology, and Genomics Core
Core Directors:
Alina Iuga, MD
Katherine Hoadley, PhD
The Tissue Procurement, Pathology, and Genomics (TPPG) Core will assist SPORE projects with expertise and guidance in pathology, genomics, and data analysis and will provide investigators with research specimens and clinical data. TPPG will provide database support for collecting, tracking, and distributing data and will ensure rigor, reproducibility, and transparency within all SPORE projects.
Integrative Quantitative Sciences Core
Core Directors:
Michael Kosorok, PhD
Naim Rashid, PhD
The Integrated Quantitative Sciences Core participates in the design of all clinical trials, animal studies, and translational research proposed in the SPORE to ensure that all relevant studies are well powered, utilize appropriate statistical methods, and are properly designed to address relevant hypotheses of study aims. In this manner the Core supports the rigor and reproducibility of all results that are generated by the SPORE, which in turn has significant impacts in the fields of public health and medicine. Expert analysis of project data and clear reporting of scientific results are of similar importance for addressing scientific hypotheses and similarly have strong implications in public health.
Developmental Research Program
Program Directors:
Albert Baldwin, PhD
Antonio Baines, PhD
The Developmental Research Program of the SToP Cancer SPORE supports developmental research into causes and treatment of pancreatic cancer. Evaluation of projects will be performed by the DRP review committee.
Career Enhancement Program
Program Directors:
Jonathan Serody, MD
Antonio Baines, PhD
The Career Enhancement Program of the SToP Cancer SPORE prepares the next generation of translational pancreatic cancer researchers.







