SPORE in Prostate Cancer
Northwestern University
Principal Investigator(s):

Sarki Abdulkadir, MD, PhD

Maha Hussain, MD, FACP, FASCO
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Targeting the MYC Pathway in Prostate Cancer
- Project 2: Re-directing the sensitivity of metastatic castration-resistant prostate cancer to immunotherapy
- Administrative, Leadership Development and Advocacy Core
- Biostatistics/Bioinformatics Core
- Biospecimen Pathology Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigator(s) Contact Information
Sarki Abdulkadir, MD, PhD
John Grayhack Professor of Urological Research
Northwestern University Feinberg School of Medicine
303 East Superior Street, Lurie 6-113, Chicago, Illinois 60611
(312) 503-5032
Maha Hussain, MD, FACP, FASCO
Genevieve Teuton Professor of Medicine
Division of Hematology/Oncology
Deputy Director
Robert H. Lurie Comprehensive Cancer Center
Northwestern University Feinberg School of Medicine
303 East Superior Street, Lurie 3-125, Chicago, Illinois 60611
(312) 908-5487
Overview
The SPORE in Prostate Cancer P50 CA180995 at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University is a collaboration with the University of Chicago Comprehensive Cancer Center and NorthShore University HealthSystem, an affiliate of the University of Chicago. Our SPORE unites basic scientists, clinicians, pathologists, biostatisticians, bioinformaticists and advocates from our academic institutions, all of whom are dedicated to advancing translational prostate cancer research. We propose two highly translational and innovative research projects that have both basic science and clinical science co-leadership:
Project 1: Targeting the MYC Pathway in Prostate Cancer (Abdulkadir, Hussain).
Project 2: Re-directing the sensitivity of metastatic castration-resistant prostate cancer to immunotherapy (Wu, Sosman).
Three core facilities support the proposed research projects: Administrative, Leadership and Advocacy (Abdulkadir, Hussain, Stadler); Biostatistics/Bioinformatics (Kocherginsky, Zhao) and Biospecimen (Yang). Internal and External Advisory Boards provide a source of scientific input to members of the SPORE team on a biannual and yearly basis, respectively. Our Advocacy Group are well established in the SPORE arena and make a valued contribution to our success. The SPORE includes Developmental Research and Career Enhancement Programs, both of which provide a source of innovation and new discoveries. All together, we anticipate that the results obtained from our research endeavors will have a significant impact on the health of patients diagnosed with prostate cancer.
Project 1: Targeting the MYC Pathway in Prostate Cancer
Project Co-Leaders:
Sarki Abdulkadir, MD, PhD (Basic Co-Leader)
Maha Hussain, MD, FACP, FASCO (Clinical Co-Leader)
MYC oncoproteins (including c-MYC, L-MYC and N-MYC) have been implicated in up to 70% of all human cancers. In prostate cancer, elevated levels of MYC protein expression are observed across all grades. In castration-resistant prostate cancer (CRPC), there is evidence of further upregulation of c-MYC levels with gene amplification occurring in 45% of cases. In late-stage, therapy-resistant neuroendocrine prostate cancer (NEPC), N-MYC is overexpressed in 40% of cases. In preclinical studies, inhibition of MYC can effectively kill CRPC and NEPC cells. A viable therapeutic strategy to inhibit MYC is therefore likely to have a significant impact on this disease and to fulfill the ongoing need for novel impactful therapies spanning the spectrum of castration resistant prostate cancer. Despite its recognition as an attractive cancer target, MYC has proved difficult to target, and there are currently no clinically viable small molecule MYC inhibitors (MYCi) available. By employing a pharmacophore-based in silico screen of a large chemical library (32 million compounds) coupled to a rapid in vivo screen in mice, we identified a series of novel small molecule inhibitors. These MYC inhibitors are highly drug-like and have shown excellent pharmacokinetic, toxicological and anti-tumor activity profiles in MYC-driven models of prostate cancer. The compounds engage MYC inside cells and inhibit MYC-driven target gene expression. Furthermore, the MYCi compounds enhance phosphorylation of MYC on threonine-58 (T58P) which promotes MYC degradation via a well-characterized ubiquitin-proteasome pathway. Treatment with MYCi impaired tumorigenicity in vitro and in vivo. The goals of this project 1 are to develop the lead MYC inhibitor, MYCi975, for clinical application in the treatment of prostate cancer and to characterize the mechanisms of MYCi-induced degradation of c-MYC and N-MYC oncoproteins. We have the following specific aims:
Aim 1 is to investigate the mechanisms of MYCi975 regulation of c-MYC and N-MYC phosphorylation and stability and the potential of MYC pT58 as a pharmacodynamic marker.
Aim 2 will assess MYCi anti-tumor efficacy and impact on pharmacodynamic biomarkers in preclinical models of c-MYC and N-MYC driven prostate cancer.
Aim 3 will seek to develop MYCi975 for use in patients by conducting formal IND-enabling toxicology studies and initiate a phase 1 trial in mCRPC patients.
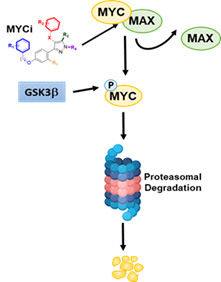
MYC inhibitor binds to MYC protein, inhibiting complex formation with MAX and recruitment to DNA binding sites. In addition, MYCi interaction with MYC protein facilitates GSK3b-mediated phosphorylation of MYC on T58. MYC pT58 is then recognized by FBW7 ubiquitin ligase for ubiquitination and proteasome-mediated degradation.
Project 2: Re-directing the sensitivity of metastatic castration-resistant prostate cancer to immunotherapy
Project Co-Leaders:
Jennifer Wu, PhD (Basic Co-Leader)
Jeffrey Sosman, MD (Clinical Co-Leader)
Limited options are available for the treatment of metastatic prostate cancer (mPC), a lethal prostate cancer. Immunotherapy has significantly improved survival in patients with a variety of solid tumors; however, it has had limited efficacy in patients with mPC. There is a lack of understanding of underlying mechanisms for this de novo resistance. In our pre-clinical studies, we have identified a novel mechanism that prostate tumors use to subvert and evade the immune system. We have found that metastatic prostate tumor cells convert the stress-induced immune stimulatory cell surface molecule, the MHC I Chain related molecule (MIC), to a highly immune suppressive soluble MIC (sMIC), through proteolytic-mediated shedding. Importantly, we find that patients with mPC have significantly elevated immune suppressive sMIC in the circulation and severely suppressed immune cell function. To overcome the immune suppression of sMIC, we have developed a first-in-class sMIC-targeting monoclonal antibody (mAb) B10G5 that has demonstrated remarkable efficacy in eliminating prostate metastasis as a single agent in preclinical models. When used in combination, B10G5 synergizes with immune checkpoint blockade. The mAb B10G5 has been optimized for human use (termed as huB10G5), proven to be safe in non-human primates (NHP) in pilot toxicity assessments, and is currently under IND-enabling studies. The goal of project 2 is to fulfill critical pre-clinical studies and to translate the B10G5 therapy into a potential therapy for treating mPC. We propose three Specific Aims:
Aim 1: to define the landscape of serum MIC levels in mPC patients with clinical characteristics and association with tumor immune modulation;
Aim 2: to determine therapeutic efficacy of targeting sMIC alone or in combination with immune checkpoint blockade (ICB) and/or AR-targeting for treatment of concurrent visceral and bone mPC;
Aim 3: To conduct a first-in-human Phase I clinical study of huB10G5 in mCRPC patients. These studies will provide us critical information for future Phase I expansion and Phase II study.
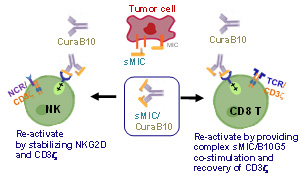
B10G5 (CuraB10) binds to sMIC and forms an immune activating agonist for NKG2D, in addition to eliminating immune suppressive effect of sMIC
Administrative, Leadership Development and Advocacy Core
Core Directors:
Sarki Abdulkadir, MD, PhD
Maha Hussain, MD, FACP, FASCO
The Admin Core is charged with oversight of the SPORE Projects and Cores with a goal to advance the best science and develop therapies that improve the outcomes of men with prostate cancer. There is a highly engaged Advocacy Group led by Daniel Shevrin, MD and Russell Szmulewitz, MD that includes a Diversity in Men’s Health program directed by Adam Murphy, MD. In addition, our unique Leadership Development Program (LDP) cultivates leadership skills that are critical for junior faculty who aspire to become future PC research leaders. The LDP will be led by Drs. Jindan Yu and Walter Stadler. Dr. Robin Leikin provides overall support to investigators and integration with the Lurie Cancer Center and the NCI.
Biostatistics/Bioinformatics Core
Core Directors:
Masha Kocherginsky, PhD
Changsheng (Jonathan) Zhao, PhD
The Biostatistics and Bioinformatics Core (Core B) consists of faculty and staff biostatisticians, bioinformaticians and statistical analysts / programmers at Northwestern University who will engage in all Core B database, bioinformatics and biostatistics efforts. Core members are involved in experimental design, planning, development and analysis of SPORE clinical trials and basic science investigations, including high-throughput sequencing studies.
Biospecimen Pathology Core
Core Director:
Ximing Yang, MD
The Biospecimen Pathology Core provides biological specimen acquisition and expert pathology consultative services to SPORE investigators. Dr. Yang works closely with SPORE investigators in the use of human and murine tissues, and analysis and interpretation of histopathology results. Core C interfaces with Biostatistics/Bioinformatics Core B in the pathologic and clinical data entry. New to Core C, senior prostate cancer investigator Dr. Jindan Yu is developing a novel methodology to measure hydroxymethylation in cfDNA isolated from patient blood (5hmC).
Developmental Research Program
Program Directors:
Sarki Abdulkadir, MD, PhD (Core Director)
Maha Hussain, MD, FACP, FASCO (Core Co-Director)
The Developmental Research Program (DRP) is an essential component of the Prostate SPORE, providing yearly funding to 4-5 projects that are selected via peer review and deemed to have promising translational potential. It is expected that DRP investigators will develop and advance their pilots sufficiently to either replace main projects that are not progressing or will secure independent funding in prostate cancer research. The DRP follows a highly structured process to solicit projects from investigators who are interested in developing projects in the area of prostate cancer, to review projects according to NIH review criteria and to monitor projects for scientific progress. Of note, both projects in the renewal emanated from pilot projects and are translating discoveries made in the lab to the clinic.
Career Enhancement Program
Program Directors:
William J. Catalona, MD (Core Director)
Simon Hayward, PhD (Core Co-Director)
The Career Enhancement Program utilizes a highly structured mechanism to select and mentor junior investigators interested in careers in prostate cancer research. The Career Enhancement Program (CEP) uses several mechanisms to recruit these investigators including emails, letters to department chairs and web site announcements. In addition, the Directors of the Robert H. Lurie Comprehensive Cancer Center and the University of Chicago Comprehensive Cancer Center advertise the CEP when recruiting junior investigators to their respective Institutions. CEP investigators are selected after careful review of their credentials, their proposed projects and their potential to develop an independent career in prostate cancer research. Career Enhancement investigators are clinicians or basic scientists from biomedical science departments at Northwestern University, University of Chicago and NorthShore University HealthSystem. The fact that the awardees come from a variety of departments provides a source of diversity and scientific expertise to members of the SPORE Program.







