Pancreas SPORE
Sloan Kettering Institute for Cancer Research
Principal Investigator:

Eileen O’Reilly, MD
- Principal Investigators Contact Information
- Overview
- Project 1: Total Neoadjuvant Therapy (TNT) for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma
- Project 2: Homologous recombination deficiency and beyond in pancreatic cancer: evaluating the regulators of response to pembrolizumab and olaparib (POLAR) from the immune and genomic perspectives
- Project 3: Recombinant Interleukin-33 Immunotherapy for Pancreatic Cancer
- Administrative Core
- Biospecimen Core
- Biostatistics Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigators Contact Information
Eileen O’Reilly, MD
Attending Physician, Member
Winthrop Rockefeller Chair
Co-Director Medical, Rubenstein Center Pancreatic Research
Memorial Sloan Kettering Cancer Center
1275 York Avenue
New York, NY 10065
Tel: 646-888-4182
Overview
The long-term translational objective of the Memorial Sloan Kettering Cancer Center (MSK) SPORE in Pancreas Cancer is to demonstrate that prospective, next-generation molecular approaches combined with state-of-the-art clinical management can improve outcomes of patients with pancreas malignancies. Specifically, we will leverage cutting-edge molecular knowledge of pancreas biology combined with clinical innovations to: 1) improve outcomes for patients with stage III and IV pancreatic ductal adenocarcinoma (PDAC) by building upon recent developments in cytotoxic and targeted therapies and apply these agents and combinations in novel disease settings; 2) build upon our extensive expertise in imaging, molecular diagnostics, biomarker development, and single-cell analyses to develop and validate prospective biomarkers of treatment response and resistance in PDAC; and 3) investigate two avenues of surmounting intrinsic immunotherapy resistance in PDAC: synthetic lethality of combination PARP inhibition and immune checkpoint blockade, and via activation of the interleukin-33 — group 2 innate lymphoid cell (IL33-ILC2) axis. We will accomplish these aims with three research projects:
Project 1: Total Neoadjuvant Therapy (TNT) for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma
Project 2: Homologous recombination deficiency and beyond in pancreatic cancer: evaluating the regulators of response to pembrolizumab and olaparib (POLAR) from the immune and genomic perspectives
Project 3: Recombinant Interleukin-33 Immunotherapy for Pancreatic Cancer
Our goals will be furthered with the support of Administrative, Biospecimen, and Biostatistics Cores, as well as Developmental Research and Career Enhancement Programs dedicated to driving innovation pancreas cancer research.
Project 1: Total Neoadjuvant Therapy (TNT) for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma
Project Co-Leaders:
Alice Wei, MD (Clinical)
Michael Berger, PhD (Basic Science)
Christopher Crane, MD (Clinical)
Project 1 will evaluate the efficacy of total neoadjuvant therapy (TNT) combining a promising triplet chemotherapy regimen (5-fluorouracil/leucovorin + liposomal-irinotecan + oxaliplatin (NALIFIROX) and sequential ablative-dose radiotherapy in patients with borderline resectable or locally advanced (BR/LA; involving major abdominal vasculature) PDAC. The primary endpoint will be event-free survival, with events defined as disease progression, recurrence after surgery (both distant or locoregional), or death on an intention to treat basis for the entire cohort. This trial aims to define TNT as a new treatment standard for BR/LA PDAC. The planned correlative studies promise to identify noninvasive biomarkers of response after TNT that may be rapidly translated to the clinic to allow adaptive disease management. Patients with BR/LA PDAC stand to benefit greatly from improvements in treatment and monitoring with the opportunity to enable curative resection.
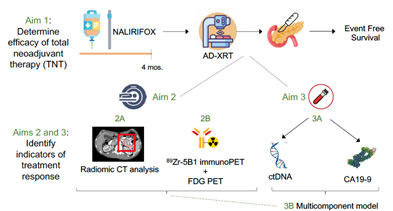
Description of Project 1.
Project 2: Homologous recombination deficiency and beyond in pancreatic cancer: evaluating the regulators of response to pembrolizumab and olaparib (POLAR) from the immune and genomic perspectives
Project Co-Leaders:
Eileen O’Reilly, MD (Clinical)
Wungki Park, MD (Clinical)
Dana Pe’er, PhD (Basic Science)
Christine Iacobuzio-Donahue, MD, PhD (Basic Science)
This project aims to expand the population for whom a synthetically lethal strategy of combination poly-ADP ribose polymerase inhibitors (PARPi) and immune checkpoint blockade therapy may apply. We hypothesize that PARPi can render PDAC immunogenic and sensitive to PD-1 blockade in a subgroup of patients with homologous recombination deficiency (HRD) beyond traditionally defined platinum-sensitive germline BRCA-mutated (gBRCA-m) PDAC. To test this hypothesis, we designed a phase-2 trial to evaluate antitumor activity of pembrolizumab and olaparib (POLAR; NCT04666740) in metastatic PDAC patients with HRD (canonical BRCA or other HR genes) and in patients with exceptional platinum response but no HR gene mutations. In this project, we will use serial biospecimens acquired from POLAR trial patients to understand the tumor genetics and features of the host and tumor immune ecosystem associated with POLAR response and resistance. The translational science in this project will provide insights regarding mechanisms of sensitivity and resistance, and immune biology that can guide future precision immunogenomic strategies to treat advanced PDAC.
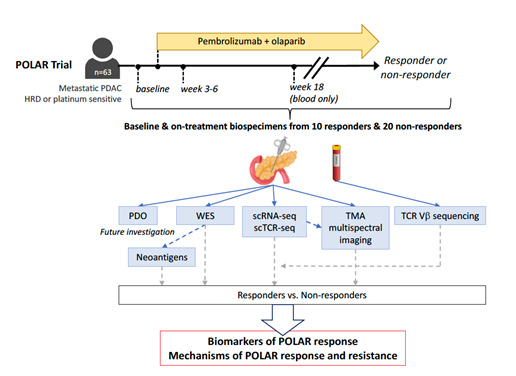
Description of Project 2.
Project 3: Recombinant Interleukin-33 Immunotherapy for Pancreatic Cancer
Project Co-Leaders:
Vinod Balachandran, MD (Basic Science)
Kenneth Yu, MD (Clinical)
This project will investigate a completely novel immunotherapy target — the IL33-ILC2 axis. Our laboratory studies identify recombinant interleukin 33 (rIL33) as a novel therapeutic cytokine that activates group 2 innate lymphoid cells (ILC2s) to stimulate de novo anti-tumor immunity in PDAC both as a monotherapy and in combination with PD-1 blockade. However, as there are currently no drugs that target the IL33-ILC2 axis, and as human PDAC tumors are immunologically heterogeneous, the appropriate drug delivery strategy and target patient cohorts to rationally translate these findings to PDAC patients are unknown. The studies proposed will identify the target patients, the mechanisms of action, and the optimal drug to inform the design of a first-in-human rIL33 clinical trial in PDAC by the end of the study period
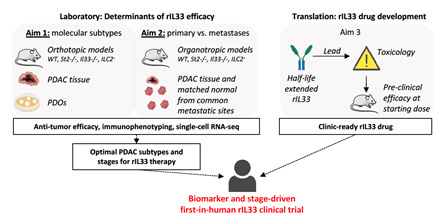
Description of Project 3.
Administrative Core
Core Directors:
Christine Iacobuzio-Donahue, MD, PhD
Eileen O’Reilly, MD
The Administrative Core will support the translational research objectives of the Memorial Sloan Kettering Cancer Center (MSK) SPORE in Pancreas Cancer by providing: (1) centralized administrative support of the daily activities of the SPORE Program, and (2) coordination for all educational and scientific activities of the SPORE, including three research projects, three cores, a Developmental Research Program (DRP), and a Career Enhancement Program (CEP).
Biospecimen Core
Core Directors:
Olca Basturk, MD
Carlie Sigel, MD
The Biospecimen Core is designed to provide expert support to the translational research efforts of the Memorial Sloan Kettering Cancer Center (MSK) SPORE in Pancreas Cancer. The objective of the Biospecimen Core is to support and facilitate the translational research efforts of the SPORE by being the central facility for all issues related to tissue and peripheral blood (including plasma and serum), including collection, annotation, processing, storage, histopathological review, tissue microdissections, generation of tissue microarrays, and execution of selected assays and their interpretation.
Biostatistics Core
Core Director:
Marinela Capanu, PhD
The Biostatistics Core will provide investigators of the Memorial Sloan Kettering Cancer Center (MSK) SPORE in Pancreas Cancer with computational and statistical support for their research efforts, including the design and analysis of laboratory experiments, molecular profiling assays, clinical trials, and imaging studies. Its support is critical for the successful completion of all research projects in the SPORE and will be available to support Career Enhancement and Developmental Research Program projects.
Developmental Research Program
Program Directors:
Jason Lewis, PhD
Diane Reidy, MD
The goals of the Developmental Research Program (DRP) of the Memorial Sloan Kettering Cancer Center (MSK) SPORE in Pancreas Cancer are to expand the breadth of the translational pancreatic cancer research base and encourage highly innovative investigations that have major potential to impact pancreatic cancer outcomes. We will support pilot projects with a clear translational focus and with potential to ultimately impact clinical care of patients with pancreatic neoplasms.
Career Enhancement Program
Program Directors:
Christine Iacobuzio-Donahue, MD, PhD
Mark Schattner, MD
The goal of the Career Enhancement Program (CEP) of the Memorial Sloan Kettering Cancer Center (MSK) SPORE in Pancreas Cancer is to support and mentor the most promising and motivated early-career investigators to prepare them for independent careers in translational research focused on pancreas neoplasms. The CEP leadership team has a strong publication record, a history of successful mentorship, and a sustained commitment to education/training. The CEP will: 1) recruit early-career investigators to the field of translational research in pancreatic cancer to enhance the overall translational research capability of the SPORE and bring fresh ideas and new talent to our program, and 2) provide these individuals the scholarly basis and mentorship needed for effective translational research in pancreatic cancer.







