MD Anderson Cancer Center Prostate Cancer SPORE
University of Texas MD Anderson Cancer Center
Principal Investigator(s):

Christopher Logothetis, MD

Timothy Thompson, PhD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Integrating Ipilimumab Immunotherapy with Approved Treatment Strategies in CRPC
- Project 2: Targeting Tumor Microenvironment-Induced Therapy Resistance in Prostate Cancer Bone Metastasis
- Project 3: Targeting Androgen Receptor and PARP for Synthetic Lethality in CRPC
- Project 4: Mitochondria, MicroRNA, and Metabolites in Predicting Aggressive Prostate Cancer
- Administrative Core
- Biostatistics and Bioinformatics Core
- Biospecimen and Pathology Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigator(s) Contact Information
Christopher Logothetis, MD
Chairman and Professor
University of Texas
M. D. Anderson Cancer Center
Department of Genitourinary Medical Oncology
1515 Holocombe Boulevard
Houston, TX 77030
(713) 792-2830
Timothy Thompson, PhD
Genitourinary Medical Oncology
M.D. Anderson Cancer Center
1515 Holcombe Blvd., Unit 0018-3
Houston, Texas 77030
(713) 792-9955
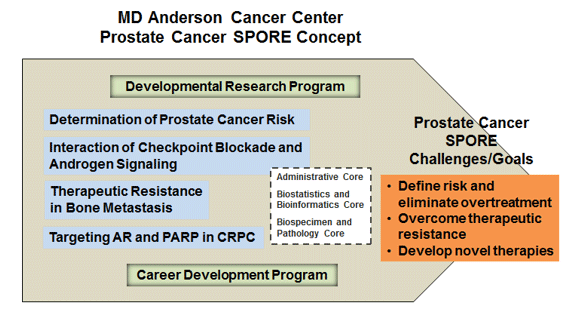
Overview
The MD Anderson Cancer Center Prostate Cancer SPORE has created a stable and dynamic infrastructure for translational research to meet and successfully address specific challenges in reducing suffering and mortality rates for men with prostate cancer. We continue to build on our capacity to conduct novel, innovative clinical trials and believe that new research projects conducted by our translational research team will yield substantial progress and meaningful changes in clinical practice. In the current proposal, we define our translational research challenges and goals as 1) quantitatively defining prostate cancer risk to eliminate the overtreatment of low-risk prostate cancer; 2) developing novel therapeutic approaches to overcoming the resistance of castration-resistant prostate cancer (CRPC) to available therapies; and 3) developing novel alternative strategies for CRPC and treatment-refractory disease. We will achieve these translational research goals by identifying and testing novel predictive and prognostic biomarkers to determine the need for therapy in patients with early-stage prostate cancer, by developing and testing novel immunotherapy for CRPC, by developing and testing a novel approach to overcoming the osteocrine-mediated resistance of prostate cancer bone metastasis to therapy, and by developing and testing lead-in combination therapy to maximize DNA damage response (DDR)-targeted therapy for CRPC. We will accomplish our goals via 4 research projects (including one population science research project), 3 support cores (an Administrative Core, a Biostatistics and Bioinformatics Core, and a Biospecimen and Pathology Core), a dynamic Developmental Research Program, and an effective Career Enhancement Program. We are optimistic that our research efforts will contribute to reductions in the incidence, morbidity, and mortality of this devastating disease by translating basic research findings into clinical practice. Our overall Specific Aims are as follows:
Aim 1. Identify and test novel predictive and prognostic biomarkers to determine the need for therapy in men with early prostate cancer. The Prostate Cancer SPORE will address the overtreatment of men with low-risk cancer through the development of predictive and prognostic markers. Dr. Xifeng Wu, an internationally recognized genetic epidemiologist, together with Drs. Jeri Kim, Jian Gu, and Jianfeng Xu (Project 4), will analyze altered energy balance and use mitochondrial DNA as predictive or prognostic markers to determine the need for therapy in men with prostate cancer detected with prostate-specific antigen screening. This project will also provide new insight into the mechanisms of prostate cancer progression that specifically lead to a lethal condition in prostate cancer patients.
Aim 2. Develop and test novel immunotherapy for CRPC. While new strategies to inhibit androgen receptor-driven prostate cancer have been developed, a major effort of our research is focused on developing therapies for CRPC, including novel immunotherapy. Dr. James Allison, an internationally recognized leader in basic and applied tumor immunology research, will team with Dr. Padmanee Sharma, a physician-scientist and past Career Development awardee, to understand and develop new immunotherapy for CRPC (Project 1).
Aim 3. Develop and test a novel approach to overcoming osteocrine-mediated therapy resistance in bone metastasis. Despite numerous FDA-approved and experimental agents in clinical trials, bone metastasis remains resistant to treatment and is the leading cause of prostate cancer mortality. Drs. Sue-Hwa Lin, Gary Gallick, and John Araujo will exploit a newly identified mechanism of therapy resistance in bone metastasis, secreted osteocrines, to develop and test a novel marker-driven therapy strategy (Project 2).
Aim 4. Develop and test a novel sequential combination therapy to maximize DDR-targeted therapy for CRPC. Drs. Timothy Thompson, Christopher Logothetis, and Paul Corn have identified a novel mechanism of progression and drug resistance that involves the upregulation of the DDR pathway. Understanding this mechanism has led to new insight into combination therapies that extend the capacity of currently available anti-androgen agents and maximize the therapeutic activities of DDR-targeting agents (Project 3).
Aim 5. Empower Prostate SPORE research and global collaborations through research Cores and Programs. The analysis of laboratory and clinical translational research findings will becoordinated by Drs. Kim-Anh Do and Bradley Broom (Biostatistics and Bioinformatics Core), who have expertise in integrative high-dimensional statistical models. The acquisition, characterization, and distribution of research tissue will be achieved by an experienced Biospecimen and Pathology Core led by Dr. Patricia Troncoso, an expert prostate pathologist. We will also develop and implement innovative translational concepts and promote the careers of translational investigators of prostate cancer through our Developmental Research Program and Career Enhancement Program, respectively.
Aim 6. Establish strategic global collaborations within the translational prostate cancer research community. Through close interactions with the NCI, the Prostate SPORE will complement other SPOREs and promote productive collaboration with other prostate cancer research initiatives internally, nationally, and internationally to benefit patients globally.
Project 1: Integrating Ipilimumab Immunotherapy with Approved Treatment Strategies in CRPC
Project Co-Leaders:
James P. Allison, PhD (Scientific Co-Leader)
Padmanee Sharma, MD, PhD (Clinical Co-Leader)
The mainstay therapy for castration-resistant prostate cancer (CRPC) consists of agents targeting the androgen receptor (AR) signaling pathway and chemotherapies to induce antitumor responses and, in select men, a vaccine and bone-targeting radiopharmaceutical therapy. The responses to these therapies tend to be short-lived. Conversely, treatment with the cytotoxic T-lymphocyte antigen 4 (CTLA-4)-targeting drug, ipilimumab, which promotes enhanced T-cell responses and generates memory immune responses, has led to the durable regression of metastatic disease and an overall survival benefit. A recent phase 3 trial demonstrated that ipilimumab elicited dramatic responses in a subset of CRPC patients and improved survival in those with favorable clinical characteristics, who also tend to have good responses to therapies targeting the AR signaling pathway. Previous studies demonstrated that the inhibition of AR signaling has positive effects on the immune system. We hypothesize that patients with cancers that regress with therapies targeting AR signaling will benefit from the addition of anti-CTLA-4 (ipilimumab) immunotherapy. In addition, given the lack of imaging capabilities to differentiate between tumor growth and tumor infiltration by immune cells as a result of immunotherapy, we propose to develop radiolabeled antibody imaging to evaluate tumor responses to immunotherapy. We aim to rationally integrate anti-CTLA-4 (ipilimumab) immunotherapy with agents targeting the AR signaling pathway to provide a durable clinical benefit with improved survival in prostate cancer patients and utilize novel imaging techniques to accurately identify tumor responses.
In this project, we will identify the molecular changes associated with the clinical outcomes of anti-CTLA-4 (ipilimumab) immunotherapy for prostate cancer by examining matched tumor and peripheral blood specimens obtained in recently completed clinical trials. We will perform in-depth examination of the specimens using immunohistochemistry, immunofluorescence, flow cytometry, gene expression studies, and serum cytokine analyses. We will prospectively evaluate the most promising molecular determinants in our novel clinical trial with the aim of linking the interactions between the immune system and the AR signaling pathway. Finally, because our group has identified other immunologic molecules that may be targeted with radiolabeled antibodies for imaging, we propose to evaluate this possibility in murine models to provide data for future clinical trials. Our proposed studies will provide data that will enable the integration of anti-CTLA-4 (ipilimumab) immunotherapy into a treatment strategy for prostate cancer that induces durable responses and improves overall survival, with the goal of curing the disease.
We propose to accomplish this in three Specific Aims:
Aim 1. Identify biological changes indicative of the mechanistic pathways that contribute to clinical outcomes in matched tumor and blood specimens obtained from prostate cancer patients given drugs targeting the AR signaling pathway plus anti-CTLA-4 (ipilimumab) immunotherapy.
Aim 2. Determine clinical outcomes after treatment with AR-targeting agents (ARN-509 + abiraterone acetate) followed by concurrent anti-CTLA-4 (ipilimumab) immunotherapy and prospectively evaluate the effectiveness of targeting selected biological pathways identified in Aim 1 to be indicative of the mechanistic pathways that contribute to clinical outcomes.
Aim 3. Determine the efficacy of targeting B7-H3 and B7-H4 with radiolabeled monoclonal antibodies as a non-invasive means to detect prostate cancer.
Project 2: Targeting Tumor Microenvironment-Induced Therapy Resistance in Prostate Cancer Bone Metastasis
Project Co-Leaders:
Sue-Hwa Lin, PhD (Scientific Co-Leader)
Gary E. Gallick, PhD (Scientific Co-Leader)
John C. Araujo, MD, PhD (Clinical Co-Leader)
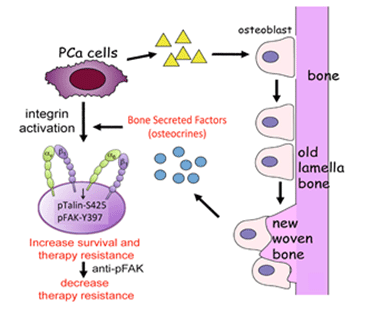
Bone metastases are the primary cause of prostate cancer mortality. Although several new targeted therapeutics for bone metastasis have improved patient survival, the disease invariably develops resistance to these therapies. Thus, there is an urgent need to improve the efficacy of therapy for prostate cancer bone metastasis. The current theory of how tumor cells acquire resistance is that tumor cells adapt in response to therapeutic agents. However, we suspect that tumor-bone interactions that occur prior to therapy may contribute to the unsatisfactory therapy outcomes. Applying agents that block or prevent this “pre-existing” resistance should increase therapy efficacy. To overcome therapy resistance, we must understand how the aberrant (woven) bone in prostate cancer bone metastases helps the tumor cells survive and resist therapy. We treated a bone-forming prostate cancer xenograft with cabozantinib, a drug currently being tested for treating bone metastasis, and found that therapy-resistant islets of viable tumor cells were mainly localized around tumor-induced woven bone. We also found that woven bone contains factors that activate survival pathways in tumor cells. (We termed these factors “osteocrines.”) Based on these findings, our overall hypothesis is that osteocrines in tumor-induced woven bone can activate survival pathways and render prostate cancer cells resistant to therapy. To test this hypothesis, we will determine: 1) the way in which osteocrines activate the signaling pathways that increase prostate cancer cells’ resistance to therapies; 2) whether these pathways can be affected by drugs or antibodies that block the identified osteocrine signaling mechanisms; and 3) whether osteocrine pathways are activated in human prostate cancer bone metastases.
The goal of this project is to determine whether blocking osteocrine-mediated therapy resistance with focal adhesion kinase (FAK) inhibition is a promising strategy for enhancing the efficacy of treatment for prostate cancer patients with bone metastasis. If the FAK inhibitors we test are proven effective in the xenograft animal model, we will test them in clinical trials. (These inhibitors are already in clinical trials in other diseases.) Our proposed study will bring a new concept, the inhibition of osteocrine-mediated therapy resistance, to bone metastasis treatment in prostate cancer patients. We predict that the inhibition of tumor microenvironment-mediated therapy resistance in combination with current therapies for prostate cancer bone metastasis will have synergistic effects in halting prostate cancer progression in bone.
The Specific Aims are as follows:
Aim 1. Determine the extent to which osteocrines confer therapy resistance through FAK activation. Because many of the osteocrines are integrin ligands that lead to FAK phosphorylation at FAK-Y397, we will study the role of pFAK-Y397 in conferring resistance to therapy in vitro and in vivo.
Aim 2. Determine the extent to which second-generation FAK inhibitors (VS-6063 or VS-4718) overcome osteocrine-induced therapy resistance in xenograft mouse models. We will 1) determine the extent to which VS-6063 or VS-4718 inhibits pFAK-Y397 in prostate cancer cells in vitro; 2) assess the pharmacodynamics of VS-6063 or VS-4718 in prostate cancer xenografts; and 3) determine whether the combination of VS-6063 or VS-4718 with chemotherapy can decrease therapy resistance in xenograft models.
Aim 3. Conduct a clinical trial to assess the toxicity and efficacy of a FAK inhibitor (VS-6063 or VS-4718) in men with treatment-refractory, bone-metastatic castration-resistant prostate cancer. We will determine 1) whether VS-6063 (defactinib) can inhibit FAK phosphorylation in metastatic castration-resistant prostate cancer; 2) whether VS-6063 has clinical benefit (i.e., whether patients with bone-metastatic prostate cancer who take the drug have prolonged progression-free survival); and 3) whether the serum or bone marrow tissue profile has a molecular signature indicating that a particular patient population is more likely to respond to FAK inhibition with VS-6063.
Project 3: Targeting Androgen Receptor and PARP for Synthetic Lethality in CRPC
Project Co-Leaders:
Timothy C. Thompson, PhD (Scientific Co-Leader)
Christopher J. Logothetis, MD (Clinical Co-Leader)
Paul Corn, MD, PhD (Clinical Co-Leader)
Metastatic castration-resistant prostate cancer (mCRPC) remains an incurable disease for which novel molecular mechanism-based combination therapy strategies are needed. We identified a DNA damage response (DDR) gene signature co-regulated by androgen receptor (AR) and c-Myb that is highly correlated with castration resistance, metastasis, and reducedoverall survival in patients with mCRPC. In this DDR gene signature, homologous recombination (HR) DNA repair genes and HR modulator (HRM) genes are highly represented. The relatively large percentage of HR/HRM genes in the DDR gene signature underscores the importance of this group of genes to prostate cancer progression. Our preliminary preclinical studies demonstrated that enzalutamide (ENZ), a second-generation anti-androgen agent that blocks androgen from binding to the AR, suppresses the expression of most HR/HRM genes and synergizes with olaparib (OLA), a poly(ADP-ribose) polymerase 1 (PARP1) inhibitor, in suppressing prostate cancer growth. OLA has been associated with synthetic lethality in multiple malignancies with BRCA1/2 or other HR gene deficiencies, and its target, PARP1, plays a crucial role in base excision repair and has been reported to function as an AR co-factor. In this project, we propose to test the hypothesis that targeting AR (with ENZ) and PARP (with OLA) in a “lead-in” strategy generates synthetic lethality in mCRPC through the ENZ-mediated downregulation of HR/HRM gene activity and the OLA-mediated suppression of PARP’s enzymatic activity in base excision repair and PARP’s cofactor role in AR transcriptional activity. The lead-in trial design will enable us to efficiently determine the clinical relevance of our biological findings by linking baseline to the sequential modulation of target genes in individual cancers. We will test this hypothesis in three Specific Aims.
Aim 1. Characterize the HR/HRM gene signature in bone marrow biopsy specimens from men with mCRPC treated with novel inhibitors of androgen signaling (ENZ and/or abiraterone).
Aim 2. Use preclinical models to further characterize the synergistic potential of and identify biomarkers predictive of response to combination therapies that co-target AR (ENZ) and PARP (OLA).
Aim 3. Conduct a clinical trial of ENZ followed by OLA in CRPC patients to achieve greater therapeutic response and correlate an ENZ-regulated HR/HRM gene signature to the therapeutic responses.
Project 4: Mitochondria, MicroRNA, and Metabolites in Predicting Aggressive Prostate Cancer
Project Co-Leaders:
Xifeng Wu, MD, PhD (Scientific Co-Leader) (MD Anderson)
Jian Gu, PhD (Scientific Co-Leader) (MD Anderson)
Jianfeng Xu, MD, PhD, (Clinical Co-Leader) (NorthShore University)
Owing to routine prostate-specific antigen screening, prostate cancer is increasingly detected at early stages, leading to a 5-year survival rate of nearly 100%. Although many screening-detected prostate cancers are indolent, about 90% of men with localized prostate cancer receive upfront aggressive treatments that often cause significant morbidity. Conversely, some patients with potentially aggressive prostate cancer who would benefit from early intervention may choose to delay treatment. This dilemma is particularly acute for patients with clinically defined intermediate-risk prostate cancer. Clinical variables alone are not sufficient to accurately differentiate aggressive and indolent diseases. Biomarkers are urgently needed to refine risk stratification. In this project, we will focus on three promising biomarkers: mitochondrial DNA (mtDNA), microRNA (miRNA), and metabolites. These multi-functional and interconnected molecules are related to obesity, an established risk factor for aggressive prostate cancer. Leveraging two of the largest prostate cancer patient cohorts in the United States, we will perform integrative analyses of these biomarkers with clinical variables to more precisely define aggressive prostate cancer. We will use knowledge gained from comparing extreme phenotypes at diagnosis (high-risk prostate cancer versus low-risk prostate cancer) to better stratify patients with clinically defined intermediate risk profiles. There are four specific aims:
Aim 1. Identify novel genetic susceptibility factors for aggressive prostate cancer at diagnosis. We will use a three-phase design: discovery, internal replication, and external validation. We have designed a custom array of about 20,000 single-nucleotide polymorphisms (SNPs), which include SNPs in miRNA regulatory pathways, SNPs in mtDNA, and SNPs that predispose to obesity and prostate cancer.
Aim 2. Identify novel intermediate biomarkers, including the mtDNA copy number in peripheral blood leukocyte DNA, circulating miRNAs, and circulating metabolites, as predictors of aggressive prostate cancer at diagnosis. We will again use a three-phase design.
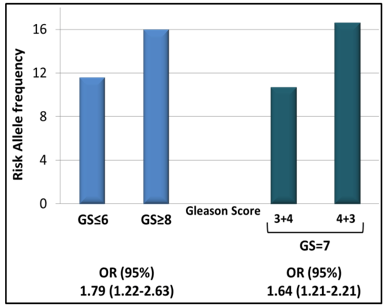
Aim 3. Test the prognostic value of validated biomarkers in special patient populations, including patients with Gleason score 7 disease, patients with localized disease receiving prostatectomy or radiotherapy, and a special patient population enrolled in an MD Anderson active surveillance study.
Aim 4. Construct multivariate prognostic nomograms that include epidemiological risk factors, clinical variables, and biomarkers from this project. We will refine clinical variables in predicting the prognosis of patients with Gleason score 7 disease and patients with localized disease receiving prostatectomy or radiotherapy. We will compare the predictive accuracy of our nomograms with existing ones that are based solely on clinical variables.
Administrative Core
Core Directors:
Christopher J. Logothetis, MD (Director)
Timothy C. Thompson, PhD (Director)
Sue-Hwa Lin, PhD (Co-Director)
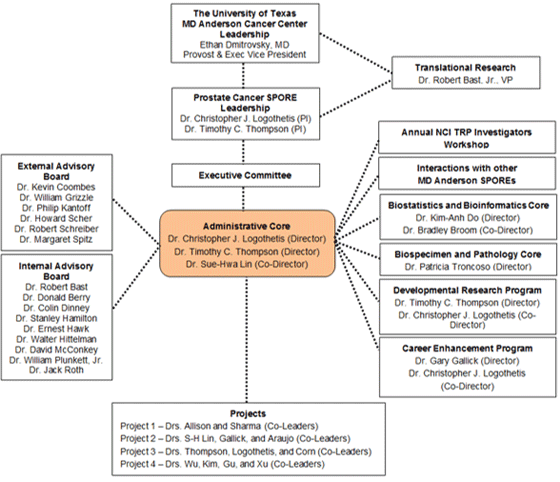
The Administrative Core provides essential support to the MD Anderson Cancer Center Prostate Cancer SPORE Principal Investigators and Investigators to maximize success. It is directed by Drs. Christopher J. Logothetis and Timothy C. Thompson and co-directed by Dr. Sue-Hwa Lin, who co-chairs the Executive Committee and provides overall supervision on 4 Projects, 2 additional Cores, the Developmental Research Program, and the Career Enhancement Program and provides scientific direction of the SPORE. The Directors and Co-Director of the Administrative Core rely on the extensive broad-based scientific, research, and SPORE experience of the Advisory Boards in critical decision-making. The success of the complex interdisciplinary research in the SPORE depends in part on the integration of diverse prostate cancer research approaches. The Administrative Core will overcome barriers to interdisciplinary collaboration and data sharing and ensure a unified translational research effort. The SPORE is founded on planning, integration, and translational research efforts supported by this Core. Its leadership and staff will be responsible for planning and monitoring scientific activities; providing scientific direction; ensuring an emphasis on translational research; ensuring interdisciplinary and inter-SPORE integration with major prostate programs within and/or outside MD Anderson and other broad translational research activities; and providing administrative and fiscal management (e.g., in terms of personnel, budget, office oversight, communication, meeting organization, manuscript preparation, progress and other reports to the National Cancer Institute [NCI] and SPORE committees, and support of Cores and Programs). The specific responsibilities of the Administrative Core are to monitor research activity and provide stable and continuous leadership and direction; promote integration, communication, and collaboration among the SPORE and collaborating investigators at MD Anderson and other Texas Medical Center institutions; monitor scientific integrity and ensure overall compliance with all institutional, state, federal, and NCI regulations and requirements, as well as assurance for data quality control for the Biostatistics and Bioinformatics and Biospecimen and Pathology Cores; provide oversight for completion of Developmental Research Program and Career Enhancement Program goals; convene, staff, and manage all necessary meetings; oversee expenditures and maintain budgets; communicate and consult with the NCI Translational Research Program Director and staff, including providing reports; increase awareness of prostate cancer research and patient advocacy in the community; address ongoing needs of minority and underserved communities in Houston and Harris County (Texas); and encourage and facilitate translational prostate cancer research by extramural groups within the region and throughout the United States.
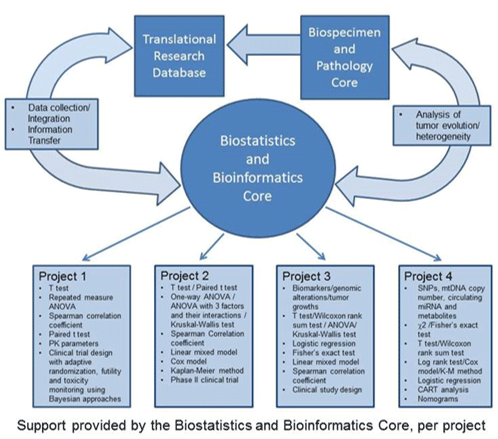
Biostatistics and Bioinformatics Core
Core Directors:
Kim-Anh Do, PhD (Director)
Bradley Broom, PhD (Co-Director)
The research proposed in the MD Anderson Cancer Center Prostate Cancer SPORE encompasses a broad range of activities, including cell line and animal model studies and clinical trials. These studies will generate many different types of data, including clinical, epidemiological, biochemical, immunohistochemical, pharmacokinetic, genotypic, and immunologic data. The Biostatistics and Bioinformatics Core for the Prostate Cancer SPORE has been a comprehensive resource for the biostatistic and bioinformatic needs that arise within the SPORE. This resource has the flexibility to match personnel with the evolving needs of the SPORE projects. We are able to incorporate sound experimental design principles within each project to enhance the interpretability of the study results, carry out data analyses using appropriate statistical methodology, and contribute to the interpretation of results via written reports and frequent interaction with project investigators. We can match Core personnel with the expertise required in a specific area of a SPORE project to efficiently meet the ongoing needs of the multiple components of the projects.
The Specific Aims of the Biostatistics and Bioinformatics Core are as follows.
Aim 1. Provide guidance in the design and conduct of clinical trials and other experiments (including high-dimensional genomic and proteomic studies) arising from the ongoing research of the SPORE.
Aim 2. Provide innovative and tailored statistical modeling, simulation techniques, and data analyses as needed for the main projects, Developmental Research Program, Career Enhancement Program, and other cores to achieve their specific aims.
Aim 3. Ensure that the results of all projects are based on well-designed experiments and are appropriately interpreted.
Aim 4. Provide guidance in the design and use of an information system to store appropriate data generated by all projects; develop integrated computational libraries and tools for producing documented, reproducible statistical and bioinformatic analyses; and support the use of these tools for analyses conducted by and on behalf of the projects.
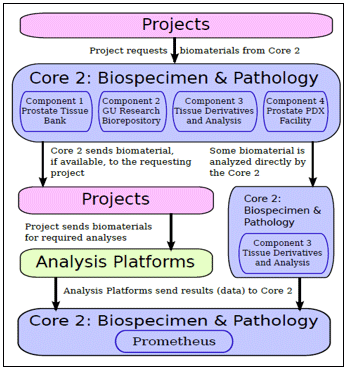
Biospecimen and Pathology Core
Core Director:
Patricia Troncoso, MD
The Biospecimen and Pathology Core will provide the biorepository, xenograft facility, pathological and technical expertise, and informatic infrastructure required to support the projects of the MD Anderson Cancer Center Prostate Cancer SPORE and ensure the achievement of their goals. These goals are 1) to develop predictive tools to better inform prostate cancer patients with low-risk disease to make evidence-based decisions and thereby eliminate overtreatment; 2) to develop a clear understanding of the molecular mechanisms of resistance to currently available, diverse therapies for castration-resistant prostate cancer (CRPC), including second-generation inhibitors of androgen signaling, immunotherapy, chemotherapy, and bone-homing radiopharmaceuticals and to develop combination therapies to overcome these resistance mechanisms; and 3) to develop novel therapeutic options for men with CRPC and treatment-refractory prostate cancer.
With its dedicated personnel, facilities, unique biospecimen resource, and prostate cancer patient-derived xenografts, the Biospecimen and Pathology Core will provide the Prostate Cancer SPORE investigators with the critical support required to conduct their translational research.
Effective interaction among the Core personnel and Prostate Cancer SPORE investigators facilitates the planning, conduct, and analysis of translational experiments to optimally use limited biospecimen resources. The participation of the Core pathologists in the selection of specimens and the interpretation of tissue-based studies promotes effective collaboration among basic researchers and clinical investigators and facilitates a uniform interpretation of the analyses. This support exists not only for Prostate Cancer SPORE investigators but also for investigators at collaborating institutions, in other SPOREs, and in broader collaborative networks dedicated to addressing the challenge of prostate cancer.
The Biospecimen and Pathology Core’s Prometheus informatics system provides the infrastructure required to integrate clinical, pathological, and translational data, thereby facilitating its interactions with the Biostatistics and Bioinformatics Core and projects and its interpretation of the results of the various analyses. The essential functions of the Biospecimen and Pathology Core are reflected in four specific aims.
Aim 1. Collect, process, annotate, characterize, store, and distribute human biospecimens related to prostate cancer.
Aim 2. Create well-characterized and quality-controlled tissue derivatives (including patient-derived xenografts) for translational research and conduct selected tissue-based studies.
Aim 3. Provide investigators with expertise to optimally select and use biospecimen resources and analytical techniques and to interpret the findings of tissue-based studies.
Aim 4. Provide an informatics solution (Prometheus) that tightly integrates biospecimen acquisition, annotation, and analysis workflows with clinical data in a secure and accessible manner.
In the pursuit of these aims, the function of the Core within the larger context of the Prostate Cancer SPORE is illustrated in the figure below.
Developmental Research Program
Program Directors:
Timothy C. Thompson, PhD (Director)
Christopher J. Logothetis, MD (Co-Director)
The goal of the Developmental Research Program (DRP) of the MD Anderson Cancer Center Prostate Cancer SPORE is to fund promising studies in translational prostate cancer research. It is directed by Dr. Timothy C. Thompson and co-directed by Dr. Christopher J. Logothetis. The DRP will emphasize the support of translational research studies that can generate clinically testable hypotheses to help reduce the incidence and mortality of prostate cancer and/or improve the quality of life of prostate cancer patients.
This program will 1) publicize the availability of start-up funding for projects in translational prostate cancer research; 2) identify projects that are innovative and have significant potential for reducing prostate cancer incidence and mortality or for increasing survival rates; 3) identify projects that complement our SPORE research focus; 4) encourage collaborations among scientists in and outside the SPORE environment to increase the potential for funding; 5) define and articulate the translational research goals and steps required to beneficially translate the research into human applications in collaboration with the Program Director and co-Director; 6) develop specific criteria for selecting projects for funding using a numerical grading mechanism based on the National Institutes of Health/National Cancer Institute application review model; 7) use the planning and evaluation process to monitor DRP progress and direction; and 8) expand the scope of the DRP with institutional funds.
Career Enhancement Program
Program Directors:
Gary E. Gallick, PhD (Director)
Christopher J. Logothetis, MD (Co-Director)
The goal of the Career Enhancement Program of the MD Anderson Cancer Center Prostate Cancer SPORE is to develop a cadre of early-career investigators dedicated to translational studies of human prostate cancer. It is directed by Dr. Gary E. Gallick and co-directed by Dr. Christopher J. Logothetis. The program will 1) foster the development of innovative entry-level scientists to the Prostate Cancer SPORE to enhance the overall translational research capability of the SPORE and bring new techniques and talent to our program; 2) help these individuals develop the intellectual and technical skills required to be productive investigators in translational prostate cancer research; 3) teach these individuals the basic principles of cancer biology not commonly included in clinical training or doctoral degree programs; and 4) guide these entry-level scientists in the development of competitive grant proposals in the area of translational prostate cancer research. The unique educational environment at MD Anderson will ensure that these goals are met.







