Route 66 Endometrial Cancer SPORE
Washington University
Principal Investigator(s):

David Mutch, MD

Doris Benbrook, PhD

Kimberly Leslie, MD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Targeting HSPA Proteins in Advanced and Recurrent Endometrial Cancer Therapy
- Project 2: Inhibiting AXL to Improve Treatment Response in Endometrial Cancer
- Project 3: Primary Prevention and Uterine Preservation in Premenopausal Women with Obesity and Endometrial Hyperplasia/Cancer
- Administrative Core
- Biospecimen, Metabolomics, and Pathology Core
- Biostatistics and Bioinformatics Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigator(s) Contact Information
David Mutch, MD
Ira C. and Judith Gall Professor
Vice Chair of Obstetrics and Gynecology
Washington University School of Medicine
66 Euclid Avenue
MSC 8064-37-9207
St. Louis, Missouri, 63110
(314) 362-3181
Doris Benbrook, PhD
PHF Presidential Professor
College of Medicine
University of Oklahoma
975 NE 10th St
BRC1217A
Oklahoma City, Oklahoma, 73104
(405)-271-5523
Kimberly Leslie, MD
Research Professor
Cancer Center- Cellular and Molecular Oncology,
University of New Mexico
MSC09 5220 1 University of New Mexico
Camino de Salud, CRT 117
Albuquerque, New Mexico, 87131
(319)-621-2145
Overview
The Route 66 Endometrial Cancer SPORE brings together interactive research teams from three institutions to create a dynamic translational research program aimed at developing and testing new strategies to prevent and treat endometrial cancer. This SPORE includes three research projects; an administrative core; a biostatistics and bioinformatics core; a biospecimens, metabolomics, and pathology core; and developmental research and career enhancement programs. The three projects, chosen and refined with extensive input from our internal and external advisory boards, are designed to have significant potential to change clinical practice within five years:
Project 1: HSPA Proteins in Advanced and Recurrent Endometrial Cancer Therapy
Project 2: Inhibiting AXL to Improve Treatment Response in Endometrial Cancer
Project 3: Improving Primary Prevention and Uterine Preservation in Premenopausal Women with Obesity and Endometrial Hyperplasia
All three projects include clinical trials and represent carefully chosen marriages between selected endometrial cancer research priorities and the strengths of Washington University in St. Louis and our collaborators at the University of New Mexico and the University of Oklahoma Health Sciences Center.
Specific Aims:
Aim 1: Test promising new therapies to treat or prevent endometrial cancer.
Aim 2: Elucidate the key biologic processes that drive endometrial cancer and develop novel biomarkers to predict development of endometrial cancer and response to therapies.
Aim 3: Leverage and enhance capacities of shared research resources.
Aim 4: Recruit and mentor new investigators and support innovative ideas in translational endometrial cancer research.
Aim 5: Facilitate collaboration of those interested in endometrial cancer research.
Aim 6: Ensure equitable enrollment in clinical trials and involvement of diverse community members and investigators in research.
In completing the work proposed, we will test new strategies to prevent or treat endometrial cancer. Future work can be directed at moving the most promising treatment approaches into larger trials. Additionally, we will obtain an unprecedented level of molecular, cellular, immunologic, and metabolomic detail regarding endometrial cancer and response to treatment, which will likely lead to development of additional novel clinical trials. Finally, by developing new ideas, investigators, and collaborations, we will expand the breadth and depth of research aimed at treating or preventing endometrial cancer.
Project 1: Targeting HSPA Proteins in Advanced and Recurrent Endometrial Cancer Therapy
Project Co-Leaders:
Kathleen Moore, MD (Clinical)
Doris Benbrook, PhD (Basic)
The incidence and mortality of endometrial cancer has increased over the past few decades, and is predicted to continue rising. There is significant need for improved therapies with reduced toxicity for women with endometrial cancers that are advanced, recurrent or refractory to standard of care. Endometrial cancer is a heterogeneous disease that has been classified into molecular profile categories with different degrees of patient prognosis. Across these categories, endometrial cancer has the highest rates of mutations in heat shock protein A (HSPA) 5, 8 and 9 genes compared to other The Cancer Genome Atlas-studied cancers. The HSPA5, HSPA8 and HSPA9 genes encode chaperone proteins, Grp78, hsc70 and mortalin, respectively, which become elevated during carcinogenesis to bind and modulate oncoproteins in a way that assures cancer cell survival. Thus, these chaperone/oncoprotein complexes represent differential targets present at higher levels in cancer cells compared to healthy cells. We developed a drug, SHetA2 (NSC 726189), which disrupts these complexes.
Specific Aims:
Aim 1: Identify mutation profiles associated with SHetA2 response and mechanism.
Aim 2: Define the utility of combining SHetA2 with paclitaxel.
Aim 3: Optimize the dosing schedules of SHetA2 with the CDK4/6 inhibitors.
Project 2: Inhibiting AXL to Improve Treatment Response in Endometrial Cancer
Project Co-Leaders:
David Mutch, MD (Clinical)
Matthew Powell, MD (Clinical)
Katherine Fuh, MD, PhD (Basic)
The prognosis for the aggressive histologies in uterine cancer patients is low. This is likely due to lack of identification of pathways specific to uterine serous cancer or high-grade endometrioid tumors specifically. Our data suggest that the AXL pathway is highly expressed in uterine serous (USC) and grade 3 endometrioid endometrial cancer (G3 EEC) is associated with worse survival. We have recently shown that high-affinity, highly-selective inhibitor of AXL, AVB-500 (now known as batiraxcept), can improve response to paclitaxel in USC and G3 EEC. Additionally, there is developing data that AXL expression is correlated with the highly glycolytic phenotype, and this correlation with glycolysis may allow us to determine which tumors can respond better to AXL inhibition. Furthermore, published data supports that AXL regulates VEGF-A, and our preclinical data supports that inhibition of AXL can improve response to the anti-angiogenic agent, bevacizumab. Our central hypothesis is that inhibiting GAS6/AXL with AVB-500 will improve response to standard of care treatment. We will expand to test this hypothesis in three specific aims.
Specific Aims:
Aim 1: Determine the safety and tolerability of combining batiraxcept with standard-of-care paclitaxel in patients with recurrent, aggressive endometrial cancer histologies (USC and G3 EEC).
Aim 2: Identify tissue and blood markers that correlate with response to treatment.
Aim 3: Determine the mechanisms by which batiraxcept improves response to the standard-of-care anti-angiogenic bevacizumab.
Impact: The clinical trial data from Aim 1 will form the foundation of a future Phase II trial of batiraxcept plus paclitaxel in USC and G3 EEC patients. The data from Exploratory Aim 2 may allow us to develop a metabolic biomarker that can predict sensitivity to this drug combination and/or provide insight into the metabolic processes involved in tumors that do not respond to this drug combination. The data from Aim 3 will provide key mechanistic data to support a future clinical trial combining batiraxcept with bevacizumab. In the long term, this work will allow us to optimize and personalize treatment for patients with aggressive uterine cancer types. Our team is well-positioned to test our central hypothesis in clinical and experimental studies.
Project 3: Primary Prevention and Uterine Preservation in Premenopausal Women with Obesity and Endometrial Hyperplasia/Cancer
Project Co-Leaders:
Andrea Hagemann, MD (Clinical)
Kimberly Leslie, MD (Basic)
Up to 90% of the ~65,000 women diagnosed with endometrial cancer each year in the U.S. are overweight or obese, and up to 60% of endometrial cancer cases are attributed to obesity. This is, in large part, because obesity promotes development of atypical endometrial hyperplasia (AEH), a precursor of grade 1 endometrial cancer. If diagnosed at one of these stages, a patient can be treated with hysterectomy, which is 100% effective in preventing/curing endometrial cancer. However, hysterectomy is often unacceptable to premenopausal women who would like to retain fertility. Instead, such patients are commonly treated with progestin, most commonly via a levonorgestrel-releasing intrauterine device. However, up to 41% of women on progestin eventually experience relapse and require a hysterectomy. Moreover, fewer than 12% of women who choose this option go on to have a live birth, likely because obesity and the commonly co-occurring insulin resistance impair fertility. As bariatric surgery can also reverse AEH and grade 1 endometrial cancer, an ideal treatment for premenopausal women desiring future fertility would be to simultaneously provide a progestin IUD along with an effective weight loss strategy.
This Early Detection, Prevention and Population Science project includes a randomized controlled trial testing the overall hypothesis that combined treatment with progestin and either therapeutic or behavioral weight loss interventions leads to greater uterine preservation than progestin use alone.
Specific Aims:
Aim 1: Determine the efficacy of progestin plus a behavioral weight loss intervention to allow uterine preservation and cancer prevention in premenopausal women with AEH or grade 1 endometrial cancer.
Aim 2: Determine the efficacy of a GLP-1R agonist plus progestin plus a behavioral weight loss intervention to allow uterine preservation and cancer prevention in premenopausal women with AEH.
Aim 3: Identify biomarkers that reflect response to progestin plus weight loss.
If this project identifies effective strategies, they can be widely implemented to allow premenopausal women with AEH or grade 1 endometrial cancer to both avoid cancer and preserve their uterus for future fertility.
Administrative Core
Core Co-Directors:
David Mutch, MD
Doris Benbrook, PhD
Kimberly Leslie, MD
The Administrative Core of the Washington University/University of Oklahoma/University of New Mexico Route 66 Endometrial Cancer SPORE will provide executive oversight, advocacy review, and administrative support for three projects, two cores (Biospecimens, Metabolomics, and Pathology Core and the Biostatistics and Bioinformatics Core), and the Developmental Research and Career Enhancement Programs. The Administrative Core leaders will receive advice from an Endometrial Cancer Equity Group, Patient Advocates, an Internal Advisory Board, and an External Advisory Board.
Specific Aims:
Aims 1: Provide administrative support for the study by providing fiscal oversight for all SPORE components including subcontracts, preparing annual progress report and interacting with relevant institutional and sponsor offices, and ensuring that all research with patient participants is ethical, respectful, safe and mindful of patients’ needs.
Aims 2: Provide organizational support for the study by coordinating meetings, committee updates, and ensuring effective communication and collaboration between the institutions.
Aims 3: Provide scientific management for the study by making decisions regarding whether to continue, discontinue, or replace full projects and whether to elevate Developmental Projects to the status of full projects; assisting investigators with preparing scholarly presentations, publications, regulatory and other documentation; and enhancing participation of underrepresented minorities in SPORE activities, both as researchers and as participants in clinical trials.
Aims 4: Provide administrative services for the clinical trials conducted within the SPORE by providing support and guidance for the proposed clinical trials and using the Washington University IRB for single site review.
Biospecimen, Metabolomics, and Pathology Core
Core Co-Directors:
Ian S. Hagemann, MD, PhD
Mark A. Watson, MD, PhD
Gary J. Patti, MD, PhD
The goals of the Biospecimen, Metabolomics, and Pathology (BMP) Core are to provide high-quality, clinically annotated biospecimens, pathology expertise, and metabolomics and spatial profiling analysis platforms to all three projects and future Developmental Research Program and Career Enhancement Program projects in the Route 66 Endometrial Cancer SPORE, and to serve as a resource to the broader endometrial cancer research community. The BMP Core is structured as a federated biospecimen resource spanning the three institutions: Washington University in St. Louis, University of New Mexico, and University of Oklahoma. The biospecimen storage and distribution component of the BMP Core leverages the extensive infrastructure of the three existing institutional Cancer Center biobanks with over 40 years of combined experience.
Specific Aims:
Aims 1: Support biospecimen collection, processing, annotation, and quality review and provide metabolomic and spatial profiling analyses for all projects in the Route 66 Endometrial Cancer SPORE.
Aims 2: Provide a high-quality, clinically annotated resource of endometrial cancer biospecimens for translational research projects both within and external to the SPORE.
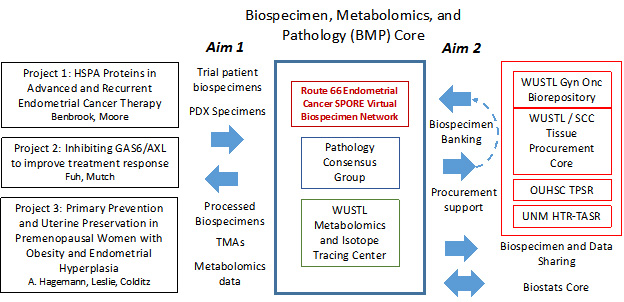
Organizational structure of the BMP Core. HTR-TASR, Human Tissue Repository and Tissue Analysis Shared Resource; OUHSC, University of Oklahoma Health Sciences Center; PDX, patient-derived xenograft; SCC, Siteman Cancer Center; TMAs, tissue microarrays; TPSR, Tissue Pathology Shared Resource; UNM, University of New Mexico; WUSTL, Washington University in St. Louis.
Biostatistics and Bioinformatics Core
Core Director:
Esther Lu, PhD
The Biostatistics and Bioinformatics Core provides state-of-the-art statistical support for all Route 66 Endometrial Cancer SPORE investigators and projects. The Core will provide statistical support on all aspects of study design, study execution and monitoring, database development and quality control, and data analysis and interpretation. Biostatistics and Bioinformatics Core members have diverse expertise, and established long-term collaborations with SPORE members at Washington University, University of Oklahoma Health Sciences Center, and University of New Mexico. Their statistical expertise will support newly developing research themes of the SPORE projects, such as Bayesian adaptive trial design, immunotherapy and target therapy trial design, biomarker-guided personalized medicine study, next-generation sequencing, and high-throughput omics data analysis, monitoring minority accrual, OnCore, REDCap, and data management/analysis for clinical studies.
Specific Aims:
Aims 1: The Core will provide biostatistics and bioinformatics collaborations for SPORE projects and Developmental Research Program studies.
Aims 2: The Core will provide biostatistics and bioinformatics support and training to early-career investigators through the Career Enhancement Program.
In achieving its aims, the Biostatistics and Bioinformatics Core will ensure that robust statistical methods and reproducible omics analyses are available to support all Route 66 Endometrial Cancer SPORE investigators.
Developmental Research Program
Program Co-Directors:
Kimberly Leslie, MD
Dineo Khabele, MD
Kathleen Moore, MD
The overall goals of the Route 66 Endometrial Cancer SPORE Developmental Research Program (DRP) are to support innovative, early-stage research in endometrial cancer, to develop pilot projects to the point of inclusion as full SPORE projects, to increase the number of investigators committed to endometrial cancer, and to diversify the workforce.
The DRP will provide one- to two-year pilot funding for projects in basic, translational, clinical, epidemiologic, and cancer prevention and control research. The DRP will be open to all three participating institutions in the SPORE.
Specific Aims:
Aim 1: Support new projects that help define the next directions in the field of endometrial cancer research.
Aim 2: Help DRP awardees achieve independent funding through competitive NIH applications, major SPORE project support, or other mechanisms.
Aim 3: Promote the participation of women, minorities, and investigators with disabilities in endometrial cancer research.
As a result of the DRP, bold new ideas in endometrial cancer research will be funded at the pilot stage and developed to lead to new treatment or prevention strategies. We fully anticipate that one or more DRP projects will be elevated to full SPORE projects in the next funding period. Additionally, new investigators will leverage DRP support to develop their projects, obtain preliminary data, and secure external funding and thus establish their careers in endometrial cancer research. Finally, established investigators who are new to the field will apply their knowledge and expertise to the field of endometrial cancer.
Career Enhancement Program
Program Directors:
David Mutch, MD
Doris Benbrook, PhD
Carolyn Muller, MD
The goal of the Career Enhancement Program (CEP) is to recruit and support early career and established investigators in translational endometrial cancer research. The CEP builds upon existing productive collaborations between outstanding researchers with expertise in translational endometrial cancer at Washington University, University of New Mexico, and University of Oklahoma. We will further enhance these collaborations by providing funding support, didactic training, career development and mentoring to new investigators in the field.
Specific Aims:
Aims 1: Recruit and support the careers of investigators new to endometrial cancer research;
Aims 2: Mentor and advise faculty researchers in translational endometrial cancer research;
Aims 3: Foster collaborations with partner institutions and other endometrial SPORE institutions, and
Aims 4: Promote diverse, equitable, and inclusive participation in endometrial cancer research.
Successful completion of these aims will support new investigators, improve our understanding of the spectrum of endometrial cancer, and develop new approaches for early detection, prevention, and treatment of this prevalent cancer. Our ultimate goal is to build a strong inclusive CEP program that mentors investigators to achieve successful careers in translational endometrial cancer research with the skills to develop an independent SPORE project and vibrant research careers.







