Head and Neck SPORE at the University of Wisconsin
University of Wisconsin
Principal Investigator:

Paul M. Harari, MD
- Principal Investigator Contact Information
- Overview
- Project 1: Priming and propagating immunity against head and neck cancer using targeted radionuclide therapy
- Project 2: Predicting Treatment Responses Using Single Cell RNA Sequencing and Bioengineered Patient-derived Organotypic Models of HNC
- Project 3: Modulation of the head and neck tumor immune microenvironment by targeting the TAM family of receptors
- Administrative Core
- Biospecimen/Pathology Core
- Biostatistics and Bioinformatics Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigators Contact Information
Paul M. Harari, MD
Jack Fowler Professor and Chairman
Department of Human Oncology
University of Wisconsin School of Medicine and Public Health
600 Highland Ave K4/438
Madison, WI 53792
Tel: 608-263-5009
Overview
The Wisconsin Head and Neck SPORE is designed to promote translational laboratory and clinical research to improve overall outcome for patients with Head and Neck Cancer (HNC). This highly collaborative research links basic scientists with HNC clinicians to advance novel treatment strategies for this complex cancer population. These patients bear a disproportionate burden from their cancers based on the critical anatomic location of the disease for which treatment can compromise speech, swallow, and breathing function, in addition to creating significant alterations in physical appearance and capacity for social interaction. Efforts to improve cure rates must be carefully balanced with efforts to reduce treatment toxicity to enable enhanced overall quality of life for patients.
The broad objectives of this SPORE are to:
- Promote multidisciplinary translational research in HNC,
- Improve overall survival and quality of life for patients with HNC,
- Incorporate new predictive models to test novel HNC treatment strategies,
- Improve understanding of how immune modulation can augment conventional and experimental treatment responses in HNC,
- Translate promising new molecules developed at the University of Wisconsin and from Industry through preclinical testing and into HNC clinical trials.
The Wisconsin HN SPORE has designed three primary research projects. Project 1 will combine targeted radionuclide therapies (TRT) with immune checkpoint inhibition (ICI) to stimulate enhanced HNC response profiles culminating in a Phase I clinical trial. Project 2 builds a powerful patient-specific bioengineered HNC model system from patient cells that incorporates components of the tumor microenvironment to more accurately predict HNC patient treatment response. The feasibility of using treatment response data from the model to inform postoperative radiation therapy will be tested in a clinical pilot study. Project 3 examines dual targeting of critical receptor tyrosine kinases Axl and MerTK to mediate changes in the immune microenvironment and thereby augment tumor response in HNC patients. The Wisconsin SPORE will support this research with three Cores (Administrative, Pathology and Biostatistics), a Career Enhancement Program and a Developmental Research Program.
Project 1: Priming and propagating immunity against head and neck cancer using targeted radionuclide therapy
Project Co-Leaders:
Zachary S. Morris, MD, PhD (Applied Co-leader)
Paul Harari, MD (Clinical Co-leader)
Jamey Weichert, PhD (Basic Co-leader)
Despite advances in treatments including the introduction of immunotherapies, metastatic or recurrent head and neck squamous cell cancers are typically incurable and, to begin changing this, we propose to develop and investigate a new treatment approach that combines the most common form of immunotherapy (immune checkpoint inhibitors) with targeted radionuclide therapy. Radiation can damage tumors in a way that renders them more responsive to immunotherapies, however conventional radiation therapy typically cannot treat all tumor sites in patients with metastatic disease because of toxicities and the inability to target small tumors that are not identified on imaging scans. Targeted radionuclide therapies go throughout the body to preferentially deliver radiation to tumors wherever they are located. In this University of Wisconsin Head and Neck SPORE grant project we will (1) determine the effect of a novel targeted radionuclide therapy on the susceptibility of head and neck squamous cell carcinoma tumor models to immune response; and (2) test the effectiveness of this type of treatment when combined with immune checkpoint blockade in order to determine which combinations are safest and most beneficial in patients with metastatic or recurrent head and neck cancer. The insights and treatment regimens developed in these studies should enable translation to advanced phase clinical testing in patients with head and neck cancers and may guide the development of similar combinations of targeted radionuclide therapies and immunotherapies for other types of metastatic cancer.
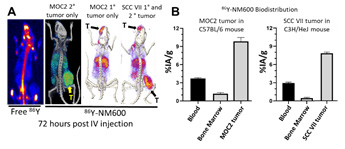
Figure A. While IV injected 86Y localizes to bone marrow of mice on PET/CT (left), 86Y-NM600 shows selective uptake in 1° and 2° MOC2 and SCC VII tumors (T, indicated by arrow) in syngeneic mice at 72 hours post-IV injection. Figure B. Measured biodistribution confirms selective uptake of NM600 in these HNSCC tumor models relative to blood and bone marrow. Preliminary dosimetry from these studies indicated that an injected activity of 50 μCi 90Y-NM600 will deliver 4 Gy to the tumor microenvironment for MOC2 and SCC VII tumors in syngeneic mice.
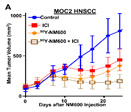
Figure C. In mice bearing MOC2 HNSCC flank tumors, we observe a cooperative effect of 50 μCi 90Y-NM600 and anti-CTLA-4 (ICI) resulting in improved tumor control.
Project 2: Predicting Treatment Responses Using Single Cell RNA Sequencing and Bioengineered Patient-derived Organotypic Models of HNC
Project Co-Leaders:
David Beebe, PhD (Basic Co-leader)
Paul M. Harari, MD (Clinical Co-leader)
This project will aim to improve prediction of treatment efficacy for patients with head and neck cancer using molecular analysis of primary tumor tissue and functional endpoints from bioengineered patient-specific in vitro models. Tumor samples from patients will be processed for analysis using single cell RNA sequencing and tissue microarrays to identify biomarkers that are associated with response to treatment with surgery and radiation or chemoradiation. Cells will be isolated from the same tumor samples and used to create patient-specific bioengineered models that recapitulate both the architecture (tumor spheroid, lymphatic and blood vasculature) and multicellular crosstalk (tumor epithelial, endothelial, lymphatic, immune, fibroblast) of the tumor microenvironment. Bioengineered models will receive the same treatment as the patient and a number of functional endpoints will be assessed to determine a metric of treatment efficacy including tumor cell proliferation and viability, DNA damage, and cytokine secretion. The predictive capacity of the models for reporting treatment efficacy will be assessed against patient outcomes. Additional experiments will compare multiple therapeutic approaches and validate that the model can report responses to targeted therapies. These findings will be translated into a pilot study investigating the feasibility of using patient-specific bioengineered models to inform radiosensitivity of patient tumors and to stratify intermediate risk HPV+ patients between radiation treatment groups (50 Gy and 60 Gy). Successful completion of this project will identify biomarkers of treatment efficacy and create a patient-specific bioengineered model that is capable of predicting treatment response profiles both for the current standard of care and for targeted systemic therapies. These data could be used to improve clinical decision making and guide more effective treatment decisions for the future in head and neck cancer.
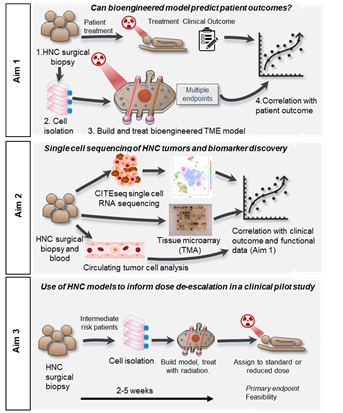
Project 2 Aims
Project 3: Modulation of the head and neck tumor immune microenvironment by targeting the TAM family of receptors
Project Co-Leaders:
Justine Yang Bruce, MD (Clinical Co-Leader)
Deric L Wheeler, PhD (Basic Co-Leader)
This project will study the cooperation of the receptor tyrosine kinases Axl and MerTK as drivers of an immunosuppressive tumor immune microenvironment (TIME) and determine if targeting tumor Axl and MerTK simultaneously can turn this suppressive TIME (cold) to an inflammatory TIME (hot). To carry out this investigation, we will utilize head and neck cancer (HNC) cell lines with genetically deleted Axl and MerTK and determine the impact on the expression of immune mediators. In addition, we will delineate the contributions of tumor- and macrophage-bound Axl and MerTK by performing a series of experiments using syngeneic mouse cancer lines with genetic deletion of Axl and MerTK and grown on an Axl/MerTK double knockout background. Further, we will perform translational studies in mice using the Axl/MerTK dual inhibitor (INCB081776) in combination with checkpoint blockade and radiation therapy (RT). Finally, this project will translate its pre-clinical findings to a clinical trial using the dual Axl/MerTK dual inhibitor INCB081776 in combination with checkpoint blockade and radiation therapy in HNC patients. Specifically, this study will perform a pilot study of INCB081776 in combination with the checkpoint inhibitor MGA-012 and external beam (RT) in patients with recurrent or metastatic HNC. Subjects will undergo baseline and on-treatment biopsies for biomarker analyses. We will measure safety and feasibility, response, and the changes in the TIME using GeoMx-DSP, scRNA sequence/CITE-seq and multispectral IF microscopy in pre- and post- treatment tumors.
Administrative Core
Core Directors:
Paul M. Harari, MD
Paul F. Lambert, PhD
Gregory Hartig, MD
The Administrative Core provides the operational structure necessary for the planning, implementation and management of all SPORE activities by providing the leadership and organizational supervision required for the efficient, progressive and successful conduct consistent with the program goals. The Administrative Core manages the oversight, monitoring and auditing required to ensure projects are meeting translational endpoints and reach a human application within five years, consistent with the SPORE mission.
Biospecimen/Pathology Core
Core Directors:
David Yang, MD
Rong Hu, MD, PhD
The Biospecimen/Pathology Core (Path Core) is dedicated to the collection, processing, preservation, characterization, annotation, and distribution of biospecimens to enhance translational research in head neck cancer (HNC). The theme of this core is to provide SPORE investigators with a reliable, high-quality, and clinically diverse source of HNC biospecimens that are exhaustively annotated with both clinical information as well as tumor specific bioinformatics. This will be achieved by enlarging the size and variety of biospecimens available to investigators, creating innovative resources such as ‘on demand’ patient derived xenografts, and augmenting biospecimen characterization including state of the art characterization of the immune background of HNC biospecimens to facilitate Immuno-oncology. The mission of the Path Core is to provide the type and quality of services that will enable HN SPORE investigators to easily initiate their translational aims and generate reliable, clinically significant findings that will impact patient care. The Path Core central goal is to meet the biospecimen needs of the HN SPORE projects and build a sustainable central organizing force for collaborative translational HNC research in the future.
Biostatistics and Bioinformatics Core
Core Directors:
Menggang Yu, PhD
Irene Ong, PhD
The objective of the Biostatistics and Bioinformatics Core (Biostats Core) of the Wisconsin Head and Neck Cancer (HNC) SPORE is to promote excellence in HNC translational research by providing dedicated and outstanding biostatistical and bioinformatic consultation and collaborative support to the HN SPORE projects, Pathology Core, Administrative Core, and other investigators whose projects are supported under the Developmental Research and Career Enhancement Programs. This support and collaboration will include laboratory, translational, and clinical-based research, focusing on translation from laboratory studies to clinical studies. The Biostats Core will achieve its objective by supporting investigators and providing statistical, informatic, and data management expertise, thus enhancing the quality and ensuring the validity of the research undertaken in the HN SPORE. The Biostats Core will provide expertise in all stages of the research: from formulation of research questions through study experimental design, data collection and management, to data analysis, interpretation, and dissemination of results through publications.
Developmental Research Program
Program Directors:
Susan Thibeault, PhD
Timothy McCulloch, MD
The Developmental Research Program (DRP) will provide Pilot Project funding to support new, innovative and high impact research in Head and Neck Cancer (HNC). The DRP funding will provide financial support to highly meritorious applications to conduct translational research focused on 1) diagnosis of HNC, 2) identification of novel biomarkers and imaging modalities in HNC or HNC progression, 3) understanding disease-causing mechanisms in HNC, 4) treatment of HNC, and 5) prevention of HNC.
The DRP will supplement and enhance an existing mechanism at the University of Wisconsin Carbone Cancer Center (UWCCC), which supports early cancer explorations and translational research collaborations through Pilot Projects and Multi-Investigator Planning Grants as part of the discovery translation mission of the UWCCC. The long term goal of the Wisconsin HN SPORE DRP will be to stimulate new research that leads to a reduction in the incidence and mortality rates of HNC. The DRP will ensure efficient, progressive and successful conduct by investigators consistent with the program goals and HN SPORE mission, designed to provide a multidisciplinary interactive environment for collaboration and the fostering of translational research ideas.
Career Enhancement Program
Program Directors:
Nadine Connor, PhD
Aaron Wieland, MD
An increase in the number of investigators who possess the research knowledge and training from the bench to the bedside is essential to our overarching goal of advancing translational research in head and neck cancer (HNC). This Career Enhancement Program (CEP) provides an outstanding structure and mechanism to cultivate the development of solid research careers for junior and mid-level faculty who strive to focus their careers on translational HNC research. The specific aim of the CEP is to provide scientists with essential training and career enhancement support to encourage the development of successful, independently funded translational research programs in the area of HNC. Our goal is to produce HNC scientists, including physician-scientists, who will meet the great need for innovation in methods to prevent and treat these devastating malignancies. The CEP will support up to three awardees at any given time. The program will continue to foster the development of knowledge, skills, professional attitudes, and experience required for successful academic careers in HNC translational research.







