Liver Cancer Disparities in Alaska Native and American Indian People
University of Washington
Principal Investigator(s):

George Ioannou, MD

William Grady, MD

Brian McMahon, MD
- Principal Investigator(s) Contact Information
- Overview
- Project 1. Novel biomarker strategies for HCC early detection in AI/AN patients
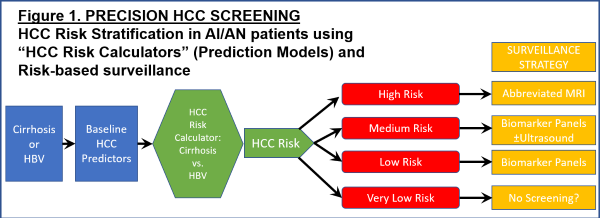
- Project 2. Risk stratification strategies and abbreviated MRI-based surveillance for early detection of HCC in high-risk AI/AN patients
- Administrative Core
- Biospecimen and Pathology Core
- Biostatistics Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigator(s) Contact Information
George Ioannou, MD
Professor of Medicine
University of Washington
1660 S. Columbian Way
Seattle, WA 98108
(206) 277-3136
William Grady, MD
Professor of Medicine
Fred Hutchinson Cancer Research Center
1100 Fairview Ave. N., G4-100, P.O. Box 19024
Seattle, WA 98109
(206) 667-1107
Brian McMahon, MD
Director Liver Disease and Hepatitis Program
Alaska Native Tribal Health Consortium
4000 Ambassador Drive
Anchorage, AK 99508
(907) 729-1560
Overview
The main focus of our Liver Cancer Disparities (Li-CAD) in AI/AN people program is to eliminate disparities in EARLY DETECTION of HCC. We believe that the most critical disparities and deficiencies in the HCC care continuum, and the greatest opportunities for improvement, lie in early detection. The overarching aim of this Program is to apply novel, innovative, translational approaches to surveillance and early detection of HCC, that are informed by unique aspects of HCC pathophysiology and epidemiology in AI/AN people, in order to eliminate disparities, improve early detection and ultimately reduce HCC-related mortality. The overarching strategy is to introduce "Precision HCC Screening" based on risk stratification and risk-based surveillance.
The Li-CAD program will achieve the following AIMS:
1. PROJECT 1. Transform biomarker-based surveillance for early detection of HCC in low and medium-risk AI/AN patients. This project will utilize discovery of novel biomarkers unique to AI/AN patients; incorporation of biomarkers into novel biomarker panels adapted for AI/AN patients; and development of innovative longitudinal (Bayesian) biomarker modeling strategies to maximize the effectiveness of biomarker-based surveillance.
2. PROJECT 2. Develop novel risk stratification strategies and test abbreviated MRI-based surveillance for early detection of HCC in high-risk AI/AN patients. This project will elucidate novel HCC pathogenetic mechanisms that are unique or particularly relevant to AN patients (HBV genotype-specific mutations); develop AI/AN-specific "HCC Risk Calculators" for HCC risk stratification and risk-based surveillance; and use these HCC Risk Calculators to identify high-risk patients for more intensive HCC surveillance strategies utilizing novel abbreviated MRI protocols, which will be tested in a small pilot and feasibility RCT.
A major asset of our Li-CAD is close integration with two major AI/AN healthcare systems that provide access to patients and biospecimens and facilitate interactions with AI/AN experts, advocates, investigators and tribal community members:
- Alaska Native Tribal Health Consortium (ANTHC), a tribally-owned health care organization providing health services to all Alaska Native people in Alaska.
- Cherokee Nation Health Service (CNHS), the largest tribally-operated health system in the United States that serves 143,761 American Indian people in Northeastern Oklahoma.
Project 1. Novel biomarker strategies for HCC early detection in AI/AN patients
Project Co-Leaders:
William Grady, MD (Basic Project Leader)
Ziding Feng, PhD (Clinical/Applied Project Lead)
Peripheral blood-based HCC biomarker panels are an essential component of early detection strategies, especially in remote AI/AN tribal communities and Indian reservations that are very far from any imaging facilities. Additionally, blood-based biomarker screening achieves greater compliance than imaging-based screening, even when imaging is readily available.
Project 1 is translational project, starting with discovery of novel HCC biomarkers based on genetic and epigenetic alterations in tumor tissue or in plasma-derived circulating free methylated DNA (cf mDNA, a particular expertise of our co-PI Grady) specifically in AI/AN patients (Specific Aim 1 [SA1]). In SA2, we will evaluate the performance of two relatively novel biomarker panels for early HCC detection (GALAD by Fujifilm-Wako and methylated DNA marker (MDM) panel by EXACT Sciences) and then improve them as necessary by adapting the GALAD algorithm to fit our AI/AN patients ("modified" GALAD) or incorporating novel methylated DNA markers that we identify in SA1. In SA3 we will develop and validate longitudinal biomarker strategies specific for AI/AN patients for HCC surveillance using AFP, GALAD or "modified" GALAD (developed in SA2). We will utilize novel longitudinal, multivariate Bayesian approaches developed by the Project Co-Leader (Dr. Ziding Feng) to capture fully the information present in multiple biomarkers modeled over time, and we will capitalize on the unique resource of our longitudinal biorepository with >40,000 biospecimens spanning many decades. The ultimate product of this bench-to-clinic translational approach will be the development of novel biomarker-based surveillance strategies that work best for AI/AN patients to improve early HCC detection.
Project 2. Risk stratification strategies and abbreviated MRI-based surveillance for early detection of HCC in high-risk AI/AN patients
Project Co-Leaders:
George Ioannou, MD, MS (Clinical/Applied Project Lead)
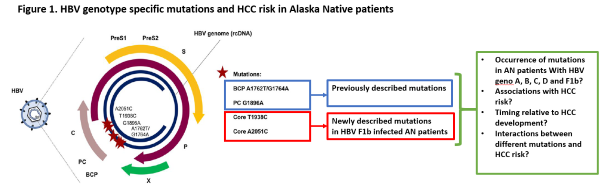
This translational project begins in SA1 by investigating the implications of a fascinating discovery made by our collaborators (Drs. McMahon and Tanaka) regarding a unique HCC pathogenetic mechanism in AI/AN HBV-infected patients: infection with genotype F1b HBV that harbors specific mutations in the core region of the virus. We will conduct human studies using our remarkable AN cohort and biorepository to understand the excess risk conferred to patients by genotype-specific mutations. In SA2 we will determine how we can combine AI/AN-specific risk factors for HCC (including HBV genotype and genotype-specific mutations that we elucidate in SA1, as well as other AI/AN-specific risk factors that we have already described) into HCC risk prediction models ("HCC Risk Calculators") that can be used to risk-stratify at risk patients for personalized, risk-based surveillance and early detection strategies, as recently described by Dr. Ioannou. This is a transformational concept in HCC surveillance and early detection, which currently employs an ineffective, one-size-fits-all strategy. We will employ traditional Cox proportional hazards modeling as well as state-of-the-art machine learning algorithms for model building, which we have recently shown to be superior for HCC risk prediction. Ultimately, one of the main aims of risk-based, personalized HCC screening is to identify very high-risk groups that would benefit from more accurate/effective surveillance strategies, which might be too expensive or resource intensive to apply to the entire population. Therefore, in SA3 we will perform a small pilot and feasibility clinical trial of abbreviated MRI (aMRI), one of the most exciting new surveillance strategies proposed for high-risk patients. If we demonstrate the feasibility of this approach, this would pave the way for larger RCTs of surveillance by aMRI in high-risk AI/AN patients in a subsequent P50 grant, adequately powered to demonstrate improvements in clinical outcomes such as early detection or receipt of curative treatments.
Administrative Core
Core Directors:
George Ioannou, MD, MS
Brian McMahon, MD
William Grady, MD
The Administrative Core of our Liver Cancer Disparities (Li-CAD) in American Indian and Alaska Native People program will serve to optimize the work of discovery conducted by the Projects and Cores through administrative support, enhancement of communication, data dissemination and structured oversight. The Administrative Core will provide guidance and enhance communication for and between the following groups:
- Li-CAD Executive Committee
- Li-CAD External and Internal Advisory Boards
- Participating Institutions (University of Washington [UW]; Fred Hutchinson Cancer Research Center [FHCRC]; Alaska Native Tribal Health Consortium [ANTHC]; Cherokee Nation Health Service [CNHS]; University of Texas Southwestern [UTSW]; Kumamoto University, Japan)
- AI/AN Leadership Council (ANTHC and CNHS)
- Discovery Team (Research Projects; Developmental Research Program; Biorepository; Biostatistics)
- Education and Advocacy Team (AI/AN Advocacy Committee, Education and Conference Committee; Career Enhancement Program)
- National Institutes of Health (NIH) and National Cancer Institute (NCI) representatives
Biospecimen and Pathology Core
Core Directors:
Matthew Yeh, MD, PhD
Brian McMahon, MD
The purpose of the Biospecimen and Pathology Core (BPC) is to provide biospecimens and pathology services to support the two planned Li-CAD P20 Projects and research studies proposed in the Developmental Research Program and to prepare for a P50 SPORE expansion of services. The BPC provides expertise in the interpretation of tissue-based assays especially in the context of biomarker studies, ensures quality standards and the ethical use of biospecimens and provides Core services in histology, digital histology, immunohistochemistry and microdissection for Li-CAD investigators.
SA1. Provide biospecimens needed for Projects 1 and 2 from AI/AN patients with liver disease, cirrhosis and HCC, as well as from appropriate non-AI/AN comparison/control patients.
SA2. Expand the biospecimen repository of American Indian patients with liver disease, cirrhosis and HCC based at Cherokee Nation Health Service (CNHS, Tahlequah, OK) and pattern it after our existing, large biorepository of Alaska Native patients at ANTHC
SA3. Provide core pathology resources in histology, digital histology, immunohistochemistry and microdissection for Li-CAD investigators
Biostatistics Core
Core Directors:
Ziding Feng, PhD
Chad He, PhD
The Biostatistics Core (BC) will support the study design, data analysis and interpretation of results for individual projects as well as facilitate interactions among all the components and cores of the Li-CAD P20. Experience has shown that involvement of biostatisticians and data scientists from the concept phase yields studies that are better designed, more likely to answer the scientific questions of interest, and, ultimately, more compelling in their conclusions. Furthermore, by combining the biostatistical services into one BC Core we will achieve greater efficiency and quality, as compared to fragmented biostatistical support for each project. The BC will also allow us to take maximum advantage of the expertise and resources of Drs. Feng and He, who are internationally recognized experts in the biostatistics of biomarker development and early cancer detection. The goal of the Biostatistics Core is to support the investigators through all phases of their studies from design to publication via the following Specific Aims:
Specific Aim 1: Provide support for STUDY DESIGN
Specific Aim 2: Provide support for ANALYSIS AND INTERPRETATION
Specific Aim 3: Promote interactions with the DRP and other Cores and Projects
Developmental Research Program
Program Directors:
Ray Yeung, MD
George Ioannou, MD, MS
The Developmental Research Program (DRP) of the Liver Cancer Disparities (Li-CAD) in American Indian/Alaska Native People Program will support early-phase projects that have the potential to open new areas of translational liver cancer research and ultimately, contribute to reducing morbidity and mortality and improving quality-of-life for AI/AN patients with hepatocellular carcinoma (HCC). Projects funded under the DRP are intended to rapidly advance a new idea or concept that has the potential to substantially impact our understanding of liver cancer to eliminate disparities and improve surveillance, early detection, and treatment of HCC in AI/AN persons and thereby reduce HCC-related mortality. The infrastructure of the DRP will establish mechanisms to quickly respond to translational research opportunities within the Li-CAD Program institutions that require support to advance hypotheses or confirm feasibility in order to justify larger resource investments. Developmental projects will include research in basic science, clinical science, and population-based studies, and will build collaborations between Li-CAD sites and other institutions with P20 Programs and SPOREs in liver cancer and disparities research.
Career Enhancement Program
Program Directors:
Ray Yeung, MD
George Ioannou, MD, MS
While not planned as an operational committee during the funding period, this application proposes development of a Career Enhancement Program that aims to provide the support, infrastructure and mentorship for junior investigators who wish to develop an independent research career focused on reducing disparities in clinical cancer outcomes among underserved populations.







