Molecularly Targeted Radiosensitization of Locally Advanced Cancers
University of Michigan Rogel Cancer Center
Principal Investigator(s):

Theodore Lawrence, MD, PhD

Meredith Morgan, PhD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Combining PARP inhibition with radiation to sensitize HR proficient pancreatic cancers to immunotherapy
- Project 2: Overcoming GBM RT-resistance
- Project 3: Credentialing CDK 4/6 inhibitors used with radiation as an effective treatment strategy in locally advanced ER+ and TNBC
- Administrative Core
- Translational Pathology Core
- Biostatistics and Computational Biology Core
- Core B: Clinical Trials Core
- Radiosensitization Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigator(s) Contact Information
Theodore Lawrence, MD, PhD
Director, U-M Comprehensive Cancer Center
Professor
Michigan Medicine
University of Michigan
1500 E Medical Center Drive
Ann Arbor, Michigan, 48109
(734) 647-9955
Meredith Morgan, PhD
Lawrence-Krause Professor of Radiation Oncology
Associate Chair of Radiation and Cancer Biology
Associate Professor
University of Michigan Rogel Cancer Center
1500 E Medical Center Drive
Ann Arbor, Michigan, 48109
(734) 647-5928
Overview
Cancer cannot be cured unless the primary tumor is cured. Systemic therapy alone can rarely cure gross disease but can significantly improve the efficacy of RT and eliminate microscopic disease. Therefore, as systemic therapies continue to improve, the need for effective local control becomes increasingly important. Although technical improvements in planning and delivery have decreased the toxicity of RT, RT dose escalation trials have, in general, failed to improve outcome. The overarching hypothesis of this SPORE proposal is that combining RT with systemic therapy that targets the molecular drivers of locally advanced cancers will improve the outcome of treatment. The goal of this SPORE is to test this hypothesis through in vitro and in vivo preclinical studies targeting key mechanisms of radiation resistance. Our studies will motivate pilot clinical trials that will define the conditions for phase II trials, yield an initial assessment of response, and test the preclinical hypotheses through correlative studies.
Specific Aims
Aim 1: Will determine if primary radiation resistance, the presence of occult metastatic disease, and a lack of immunogenicity can be targeted by increasing radiation-induced DNA damage using a PARP inhibitor with immune checkpoint blockade (ICB). We test this strategy in preclinical and clinical studies combining olaparib, RT, and durvalumab in pancreatic cancer.
Aim 2: Will determine if the radiation resistance due to unique tumor metabolism, specifically, elevated purine levels, can be targeted. We test this hypothesis in preclinical and clinical studies of glioblastoma using the purine depleting CNS-penetrant FDA approved agent mycophenolate mofetil (MMF).
Aim 3: Will determine if the radiation resistance due to aberrant cell cycle control and activated DNA repair can be targeted in tumor cells with intact retinoblastoma protein (RB) using a CDK4/6 inhibitor. We test this hypothesis in preclinical and clinical studies in patients with locally advanced ER+ or triple negative breast cancers with intact RB using RT with concurrent abemaciclib.
We have a strong history of vertical translation: our prior early phase trials in these three diseases have progressed to multi-institutional trials.
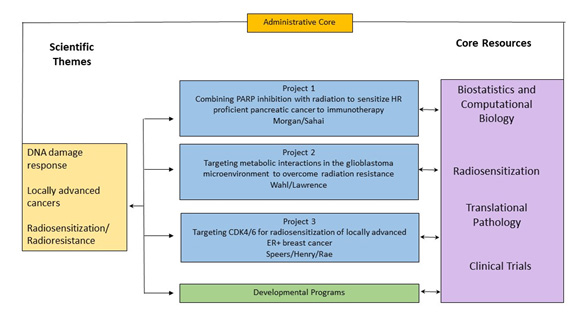
Figure: SPORE schema.
Project 1: Combining PARP inhibition with radiation to sensitize HR proficient pancreatic cancers to immunotherapy
Project Co-Leaders:
Meredith Morgan, PhD (Basic)
Vaibhav Sahai, MBBS (Clinical/Applied)
The overall goal of this project is to preclinically develop a strategy combining PARP inhibitors with radiation for sensitizing PDAC to ICB by promoting tumor cell DNA damage/replication stress and immunogenicity that will be translated to a clinical trial in LAPC patients.
Specific Aims
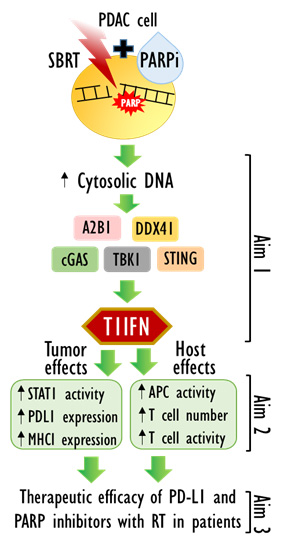
Aim 1: Will elucidate novel mechanisms of innate immune modulation and their T1IFN-dependent phenotypic consequences by PARP inhibitors with radiation in PDAC cells. We anticipate defining novel pattern recognition receptor pathways initiated by olaparib and radiation-induced DNA damage that result in increased tumor innate immunity.
Aim 2: Will determine the therapeutic benefit, immune contribution, and molecular endpoints of combined therapy with PARP inhibitor, radiation and ICB in PDAC. We expect to achieve a favorable therapeutic index with SBRT under an optimized schedule that is accompanied by both local and systemic tumor responses as well as both innate and adaptive immune responses. This aim will inform the design of our clinical trial in Aim 3.
Aim 3: Is a clinical trial of olaparib, radiation, and durvalumab in patients with LAPC. Improvements in outcome of treatment of LAPC require control of both gross local disease and micrometastases. Our preclinical studies combining olaparib, radiation and anti-PD-L1 show durable tumor growth inhibition supporting their clinical investigation. We will dose escalate olaparib with the combination of SBRT n and the PD-L1 blocking antibody durvalumab in patients with LAPC. For olaparib dose escalation, we will use the Time-to-Event Continual Reassessment Method (TiTE-CRM) that gives a better estimate of the phase II dose than the standard 3+3 design and also permits estimation of efficacy, similar to a phase I/II trial. We will assess markers of innate and adaptive immunity (identified in Aim 2B) in tumor biopsies as well as circulating tumor and immune cells, respectively. We anticipate that we will achieve a safe and efficacious combination of olaparib with radiation and durvalumab that stimulates immunogenicity and motivates a randomized phase 2 trial to ultimately improve survival of patients with LAPC.
Overview of Project 1 aims.
Project 2: Overcoming GBM RT-resistance
Project Co-Leaders:
Daniel Wahl, MD (Basic)
Theodore Lawrence, MD, PhD (Clinical/Applied)
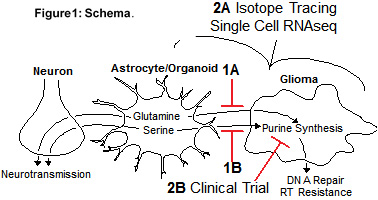
Radiation is a cornerstone treatment for glioblastoma but tumors inevitably recur due to radiation resistance. We found that non-malignant cells in the glioblastoma microenvironment mediate radiation resistance by stimulating purine metabolism in cancer cells. This project will define how metabolites from non-malignant cells promote glioblastoma purine metabolism and how interrupting regulation could improve outcomes for patients. Our central hypothesis is that GBMs co-opt physiologic astrocyte glutamine and serine secretion to fuel purine synthesis, which mediates RT resistance. Our preliminary data support this hypothesis, and we will further test this hypothesis through two specific aims:
Specific Aims
Aim 1: To determine the metabolic mechanisms by which non-malignant cells promote GBM purine synthesis and RT resistance. We will determine how astrocyte or organoid-secreted glutamine (Aim 1A) and serine (Aim 1B) regulate glioma cell de novo purine synthesis, DNA repair and RT resistance. Each sub aim will utilize astrocytes, organoids, glioma neurospheres and orthotopic GBM xenograft models. We hypothesize that inhibiting astrocyte/organoid glutamine and serine synthesis will decrease glioma cell purine metabolism, DNA repair and RT resistance.
Aim 2: To measure purine metabolism and perform a phase I clinical trial combining MMF with standard therapy in patients with GBM. We hypothesize that de novo purine synthesis will be inactive in non-neoplastic brain but elevated in GBM. We expect that higher activity of de novo purine synthesis in GBM will correlate with increased astrocyte content and benefit from MMF. We believe that the enzymes required to synthesize serine and glutamine will be elevated in non-malignant astrocytes while those involved in de novo purine synthesis will be elevated in GBM cells.
Project 3: Credentialing CDK 4/6 inhibitors used with radiation as an effective treatment strategy in locally advanced ER+ and TNBC
Project Co-Leaders:
James Rae, PhD (Basic)
Corey Speers, MD (Basic)
N. Lynn Henry, MD (Clinical/Applied)
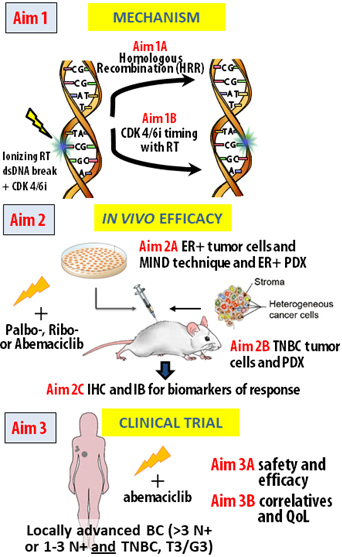
Based on preliminary findings, we hypothesize that CDK4/6 inhibition combined with RT causes clinically meaningful radiosensitization in an RB1-dependent manner through a decrease in HRR. Furthermore, we hypothesize that the combination of abemaciclib with RT is safe, tolerable, and effective in women at high risk of local recurrence of BC. We will test as follows:
Specific Aims
Aim 1: Determine the schedule of treatment and mechanism of CDK4/6i-mediated radiosensitization in ER+ and TNBC models and dependence on RB1 status. We anticipate establishment of the mechanism of CDK4/6i-mediated radiosensitization, the most effective treatment schedule of combined therapy, and dependence on RB1, thus informing both the animal studies and appropriate patient selection in future studies.
Aim 2: Determine the efficacy of CDK4/6 inhibition in combination with ionizing RT in in vivo models of ER+ and TNBC. In Aim 2 we will determine the degree of radiosensitization of orthotopic xenograft cell line and PDX models of ER+ and TNBC in vivo after treatment with RT+ palbo-, ribo-, or abemaciclib in RB1 wt and RB1 mutant PDXs. Using tumors from these xenograft studies, we will perform immunohistochemistry (IHC) and western blotting to evaluate changes in HR, NHEJ, apoptosis, and RB1 status. We anticipate confirming the mechanistic findings in Aim 1 and confirm radiosensitization in RB1 intact ER+ and TNBC models in vivo.
Aim 3: Conduct a clinical trial of abemaciclib in combination with RT in patients with locally advanced BC (LABC) at high risk for local recurrence. We will perform a Phase IB dose escalation trial with abemaciclib combined with radiation in patients with LABC, to determine the recommended phase II dose and to estimate efficacy. We will also collect liquid biopsies to assess ctDNA kinetic changes, determine RB1 status in pretreatment biopsies, measure pharmacodynamic endpoints of abemaciclib-mediated target inhibition and DNA damage identified in Aims 1 and 2, and serially assess patient-reported symptoms and quality of life (QoL). We anticipate that we will achieve a dose of abemaciclib in combination with radiation that would be predicted to inhibit CDK4/6 and that is safe and tolerable, supporting the conduct of a larger Phase II efficacy trial.
Administrative Core
Core Directors:
Theodore S Lawrence, MD, PhD
Meredith Morgan, PhD
The Administrative Core of the University of Michigan Radiosensitization SPORE supports all projects, cores, and developmental programs of the SPORE. The Administrative Core fosters in-depth communication among all SPORE components to enhance research integration, provide critical scientific feedback, evaluate scientific progress, plan for future opportunities, and assert translational focus. The Core communicates with the National Cancer Institute staff and helps its scientists disseminate their findings rapidly to the public. The Administrative Core serves the following functions:
Specific Aims
Aim 1: Leadership
Aim 2: Scientific management
Aim 3: Administrative management
Aim 4: Integration into Cancer Center and institutional scientific programs: Ensures that the SPORE integrates with the U-M Rogel Cancer Center; the University organization overseeing translational research, the Michigan Institute for Clinical and Health Research (MICHR); and individual University of Michigan Schools’ offices of research programs and resources.
Aim 5: Access to cancer patient and risk populations
Aim 6: Increasing representation of underserved minorities in SPORE clinical research
Aim 7: Enhancing diversity in the SPORE research team
Aim 8: Managing SPORE data: Works collaboratively with the Biostatistics and Computational Biology Core to ensure it integrates into institutional informatics resources and is compatible with NIH/NCI data management resources.
Aim 9: Providing access to SPORE data and bio samples
Aim 10: Planning and Evaluation Activities
Translational Pathology Core
Core Directors:
Thomas Giordano, MD, PhD
Dafydd Thomas, MD, PhD
The Translational Pathology Core (TPC) will oversee the procurement, processing, management, and distribution of biospecimens obtained from SPORE patients. The core will also generate histological and immunohistochemical data for SPORE projects, as well as provide diagnostic expertise related to the interpretation of these data. The TPC will serve the three projects of the Radiosensitization SPORE as well as future career enhancement or developmental research projects.
Specific Aims
Aim 1: To procure, process, archive, and distribute tissues to the primary projects of the Radiosensitization SPORE.
Aim 2: To provide histological and immunohistochemical processing of tissue samples from the Radiosensitization SPORE preclinical animal models and clinical trial patients.
Aim 3: To provide expert interpretation and annotation of histological and immunohistochemical results of tissue samples from the Radiosensitization SPORE preclinical animal models and clinical trial patients.
Aim 4: To provide services related to cellular composition of tissue specimens generated by the Radiosensitization SPORE by our multiplex immunohistochemical service/platform as well as by partnership with the Immune Monitoring Shared Resource and the Advanced Genomics Core.
Biostatistics and Computational Biology Core
Core Co-Directors:
Matthew Schipper, PhD
Arvind Rao, PhD
The Biostatistics and Computational Biology Core provides comprehensive biostatistical and computational biology support to SPORE members. The core will work closely with investigators to provide statistically rigorous and efficient study designs, modern and robust data analysis as well as standardized bioinformatics analysis pipelines for the analysis of CyTOF, single cell RNAseq and multiplex IHC data. Core members will consult with investigators at each stage of research including planning experiments, conducting the clinical trials, analyzing the data and interpretating and disseminating of results. The core will support data flow and warehousing within the SPORE as well as with entities outside of the SPORE. The specific aims of the biostatistics and computational biology core are to:
Specific Aims
Aim 1: Collaborate with investigators on the design of clinical trials and pre-clinical experiments.
Aim 2: Provide robust and efficient statistical analysis of scientific data for all the projects.
Aim 3: Provide comprehensive bioinformatics support for SPORE investigators.
Aim 4: Support data management within the SPORE involving both the coordination of curated, model ready data exchange among the Projects, Cores, and Programs and use of final data sets with outside collaborators.
Core B: Clinical Trials Core
Core Co-Directors:
Scott Schuetze, MD, PhD
Kyle Cuneo, MD
The Clinical Trials Core (CTC) will prioritize and ensure development, implementation, conduct and completion of high-quality clinical trials to support the SPORE. The complexity of translational research trials in humans requires specialized research coordination skills incremental to services provided by the U-M Rogel Cancer Center and Medical School Oncology-Clinical Trials Support Unit (O-CTSU). The O-CTSU supports regulatory compliance (adverse event reporting, submission of annual renewals, communication with regulatory entities, and maintenance of regulatory documents) and data management services. The CTC supports clinical protocol development, patient recruitment and retention, logistical coordination, and bio sample management. The CTC will also interact with the O-CTSU to monitor data management for the SPORE clinical trials.
Specific Aims
Aim 1: Provide experienced leadership to move basic discoveries quickly to clinical trials.
Aim 2: Contribute logistical support to develop, implement and conduct clinical trials.
Aim 3: Institute strategies to ensure successful completion of SPORE clinical trials.
Aim 4: Establish processes to assure Good Clinical Practice.
Aim 5: Educate and mentor all investigators in translation of their discoveries into clinical trials.
Aim 6: Integrate with the SPORE Cores and other institutional resources.
Aim 7: Oversee data management for and data sharing of the clinical research data collected in the SPORE clinical trials.
Radiosensitization Core
Core Directors:
Alnawaz Rehemtulla, PhD
Judith Leopold, PhD
James Balter, PhD
The Radiosensitization Core will provide each of the research projects scientific expertise, technical knowhow, and instrumentation for radiation delivery in mouse and cell culture models under conditions that best approximate the clinical setting to provide confidence that the derived research result findings are more likely to impact the management of patients with cancer. The Core will leverage state-of-the-art technological advances in radiation physics and treatment planning, imaging for monitoring of tumor burden and treatment efficacy, mouse models of cancer and drug pharmacodynamics into a single integrated research component. To enable efficient execution of in-vitro and in-vivo studies with scientific rigor and translational relevance, we leverage expertise at U-M Rogel in each discipline for evaluation of each disease-specific radiosensitization strategy in pre-clinical model systems.
The goal of the Radiosensitization Core is to provide the scientific expertise, technical knowledge and standard operating procedures for the proposed studies and use of instrumentation.
Specific Aims
Aim 1: Deliver radiation therapy with high precision in mouse models using image guidance.
Aim 2: Conduct mechanistic studies to delineate the functional basis for radiosensitizing activity of each targeted agent.
Aim 3: Optimize dose and schedule of combination therapies for informed translation into humans.
Developmental Research Program
Program Directors:
Eric Fearon, MD, PhD
Theodore Lawrence, MD, PhD
The Developmental Research Program (DRP) in this SPORE will support high-risk/high-yield cancer research by early-to-mid-career investigators and seasoned investigators focused on combining novel agents with radiation therapy. A chief goal in the research projects will be the generation of key preliminary data and the achievement of milestones that support submission of an R01 application or an equivalent proposal (e.g., project in a future SPORE application). Another high priority area for support by the DRP will be pre- clinical or early-stage clinical projects that will generate key proof-of-concept results necessary prior to more extensive human application studies. The DRP will add value to the SPORE by its ability to stimulate the development of pilot projects via research project support. The DRP will also add value by its ability to engage and support faculty via mentoring opportunities and collaborative interactions with SPORE investigators and through the critical intellectual and technical support and resources provided by our SPORE Cores. We fully expect the DRP will prove a vital mechanism for the SPORE to make strides towards improving treatment options and outcomes for patients with unresectable solid tumors.
Career Enhancement Program
Program Directors:
Mats Ljungman, PhD
Jennifer Shah, MD
The Career Enhancement Program (CEP) will identify and foster a diverse pool of investigators focused on studies of molecularly targeted radiosensitization. The CEP identifies early career investigators who demonstrate excellent progress towards independence, established investigators interested in transitioning their research to translation of radiation cancer biology to patient care, and senior investigators with cancer research experience who desire to develop additional expertise in translational science. A high priority of the CEP is to identify, support and mentor members of groups traditionally under-represented in academic health sciences. The SPORE will develop relationships to enable identification and encourage proposals from groups traditionally underrepresented in academic health sciences from nearby institutions serving minority populations. Mentoring teams will include experienced mentors who will also be provided with formal training in both mentorship and sponsorship. The CEP will support participants’ professional development in areas like clinical trial design and grant writing; it will also offer real-world opportunities for honing presentation and networking skills in the SPORE annual retreats. The CEP curriculum will target skills in areas like negotiation, work-life integration, and leadership development, encouraging peer mentorship in addition to the other forms of mentorship established in the program. Impact will be carefully measured and evaluated.







