Roswell Park-University of Chicago Ovarian Cancer SPORE
Roswell Park Comprehensive Cancer Center
Principal Investigator(s):

Kirsten Moysich, PhD

Kunle Odunsi, MD, PhD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Reprogramming the immunosuppressive ovarian TME with OV and a CXCR4 antagonist
- Project 2: Synchronizing immunopeptidome of EOC-pulsed DC with tumor cells to ensure expansion of tumor-reactive T cells
- Administration Core
- Biostatistics and Bioinformatics Core
- Biospecimen and Pathology Core
- Immunogenomics Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigator(s) Contact Information
Kirsten Moysich, PhD
Distinguished Member and Professor of Oncology
Department of Cancer Prevention and Control
Roswell Park Comprehensive Cancer Center
Elm & Carlton Streets
Buffalo, NY 14263
(716) 845-8004
Kunle Odunsi, MD, PhD
Director, University of Chicago Medicine Comprehensive Cancer Center
Dean for Oncology, Biological Sciences Div.
University of Chicago Medicine Comprehensive Cancer Center
5841 S. Maryland Avenue, MC 1140
Chicago, IL 60637
(773) 702-5769
Overview
The overarching goal of the renewed Roswell Park-University of Chicago Ovarian Cancer SPORE is to conduct multidisciplinary, mechanism-based and collaborative translational research that will have the highest possible impact for women with ovarian cancer. Because immunotherapies have met with only modest success in ovarian cancer patients, we continue to uniquely focus on novel strategies for generating effective anti-tumor immunity by unraveling immune-resistance mechanisms and identifying novel proteogenomic biomarkers of responsiveness. After significant planning and guidance by our Internal and External Advisory Boards and Patient Advocate Committee, we have leveraged our highly successful DRP and CEP to propose two bi-directional translational IRPs addressing basic and clinical research questions of importance in ovarian cancer. The new IRP1 evolved as a result of a DRP award, and the new IRP2 developed from a CEP award. IRP1 will test an oncolytic virus armed with a CXCR4 antagonist in combination with PDL1 blockade to abrogate tumor immune suppression and limit T cell exhaustion in a randomized Phase I/II clinical trial. IRP2 addresses the completely novel concept of identifying mismatch between immunopeptidomes of ovarian cancer cells versus dendritic cells and leveraging a computational approach of bypassing such mismatch. IRP1 commences with a planned clinical trial; the Phase I/II clinical trial in IRP2 will commence in year 3, following preclinical, IND-enabling translational studies. The program also continues to expand opportunities for new avenues of ovarian cancer translational research via its successful DRP and CEP. The four highly integrated, interconnected shared resource cores — Administration, Biospecimen & Pathology, Biostatistics & Bioinformatics, and Immunogenomics — bring innovative technology and resources to the SPORE and do not duplicate pre-existing shared resources available at Roswell Park or the University of Chicago. This application is strongly supported by over $3.6MM of institutional commitment to ensure success in its goal of conducting highly innovative translational research that changes the clinical practice paradigm in ovarian cancer.
Project 1: Reprogramming the immunosuppressive ovarian TME with OV and a CXCR4 antagonist
Project Co-Leaders:
Kunle Odunsi, MD, PhD (Clinical Co-Leader)
Danuta Kozbor, PhD (Basic Co-Leader)
The goal of our studies is to generate efficacious tumor-specific T cell responses in patients with chemotherapy-resistant high-grade serous ovarian cancer (HGSOC). Key stumbling blocks underpinning immunotherapy resistance against HGSOC include: (i) insufficient expansion of tumor antigen-specific T cells, (ii) recruitment of T regulatory and myeloid-derived suppressor cells via tumor CXCL12 production, (iii) exhaustion of tumor-infiltrating T lymphocytes (TILs) associated with upregulation of PD1, (iv) low tumor immunogenicity due to reduced mutation load and IFNβ production, (viii) insufficient recruitment of intratumoral dendritic cells, and (ix) disorganized tumor vasculature incapable of supporting TIL trafficking. Herein, we propose to target these immune resistance mechanisms using an innovative, clinically translatable viral oncotherapy combination for effective direct oncolysis and reprogramming antitumor immunity.
The scientific premise of this project stems from our discovery that blockade of the CXCL12 chemokine/CXCR4 receptor axis in chemotherapy-resistant ovarian cancer by intraperitoneal delivery of an oncolytic vaccinia virus expressing a CXCR4 antagonist (OVV-CXCR4-A-Fc) in combination with liposomal doxorubicin yields a significant therapeutic impact by reversing the immunosuppressive tumor microenvironment (TME) and stimulating vigorous T cell responses. Our proposed mechanistic studies to be conducted in a phase I/II clinical trial will test the hypothesis that, in patients receiving liposomal doxorubicin for platinum-resistant/refractory HGSOC, in vivo tumor destruction by OVV-CXCR4-A-Fc will provide clinical benefit by (i) abrogating tumor immune suppression, (ii) promoting the accumulation of TILs, and (iii) when combined with PDL1 blockade, limiting T cell exhaustion. The approach is to first determine whether intraperitoneal OVV-CXCR4-A-Fc safely transforms the ovarian TME from tolerogenic to immunogenic in a first-in-human clinical trial in patients with platinum-resistant/refractory HGSOC. Second, we will establish whether OVV-CXCR4-A-Fc combined with anti-PDL1 antibody treatment is safe and clinically efficacious. Third, we will test whether the combinatorial regimen generates functionally distinct tumor-specific CD8+ and CD4+ effector/memory cells. Finally, we plan to uncover the molecular mechanisms by which OVV-CXCR4-A-Fc overcomes the ovarian tumor “vascular checkpoint” to enhance T cell migration and trafficking.
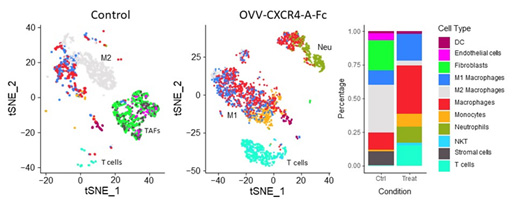
Graphical summary of reprograming the ovarian TME by treatment with CXCR4-A-Fc-armed oncolytic virotherapy analyzed by scRNAseqCell types on TSNE plots are highlighted by matching colors on the data panel.
Project 2: Synchronizing immunopeptidome of EOC-pulsed DC with tumor cells to ensure expansion of tumor-reactive T cells
Project Co-Leaders:
Pawel Kalinski, MD, PhD (Applied Co-Leader)
Song Liu, PhD (Basic Co-Leader)
Kunle Odunsi, MD, PhD (Clinical Co-Leader)
Project 2 tests a new immunization strategy to enhance the induction of cytotoxic lymphocytes (CTLs) against multiple patient-specific epitopes, by targeting ovarian cancer (OvCa) cells and dendritic cells (DCs). We will compare the immunopeptidomes on OvCa cells and two populations of DC specialized in CTL induction: ex vivo generated alpha-type-1-polarized (αDC1s) and endogenous conventional DCs (cDC1s) which share the inflammatory BATF3/IRF8 phenotype and elevated ability to cross-present multiple cancer-cell-associated antigens to CD8+ T cells.
Combining our unique in vitro sensitization and bioinformatics approaches, we will test the overall hypothesis that mismatch between immunopeptidomes of OvCa cells and DCs presenting antigens from cancer cells limits therapeutic effectiveness of spontaneous and vaccination-induced CTL responses. We further hypothesize that αDC1s loaded with synthetic patient-specific neoantigen peptides will bypass such mismatch, inducing CTLs particularly effective in killing OvCa tumors. We propose the following three Aims:
Specific Aim 1: Compare the antigenic specificity of human CD8+ T cells induced by DCs loaded with autologous OvCa cells, tumor-eluted peptides and patient-specific neoantigen peptides identified in silico. We hypothesize that CTLs induced by autologous cancer cell-loaded αDC1s or cDC1s contain many CTLs which are irrelevant for tumor recognition, which can be corrected by loading DCs with synthetic neoantigen peptides specific to each patient’s OvCa cells.
Specific Aim 2: Evaluate immunopeptidome differences between OvCa cells and tumor-loaded αDC1s and endogenous cDC1s and test the feasibility of their adjustment. We hypothesize that immune adjuvants and inflammatory mediators can be used to modulate APM patterns and immunopeptidomes of DCs and OvCa cells, to enhance the antigenic match between arising CTLs and autologous OvCa cells.
Specific Aim 3: Determine the feasibility, safety and clinical efficacy of αDC1 vaccines loaded with patient-specific neoantigen peptides combined with PD-1 blockade. Guided by the results of Aim 2, the patients may also receive systemic immune modulation to increase the visibility of their own OvCa cells to αDC1-induced CTLs and reduce immune suppression.
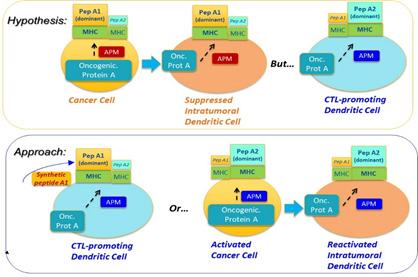
Figure 1. Central Concept: Synchronizing the immunopeptidomes of activated DCs and cancer cells.
Administration Core
Core Directors:
Kirsten Moysich, PhD, MS
Kunle Odunsi, MD, PhD
The overall objective of the Roswell Park-University of Chicago Ovarian Cancer SPORE Administration Core is to provide leadership, direction, scientific and fiscal oversight, and administrative services to enhance research productivity and maintain a stimulating research environment conducive to translational research in ovarian cancer. The Administration Core will catalyze, oversee, and support interactions amongst SPORE components, external and internal collaborators, other SPOREs, patient advocates, and the National Cancer Institute. In order to achieve the overall objective of managing SPORE resources and maintaining high quality in all components, the Administration Core will be organized into four main operational areas: 1) Leadership; 2) Administrative Management; 3) Clinical Trials, Data, and Communications Management; and 4) Scientific Management. Management and support are accomplished through an organizational framework that takes advantage of highly experienced leadership that is integrated within Roswell Park Comprehensive Cancer Center and the University of Chicago Comprehensive Cancer Center, an oversight executive committee for planning and evaluation of activities, and scientific and patient advocate advisors. The functions performed by the Administration Core will provide greater integration and efficiency across the program and will result in lower operating costs for each individual SPORE component.
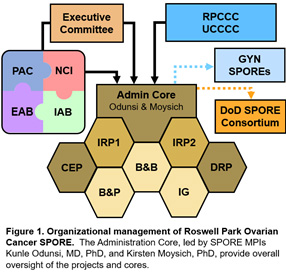
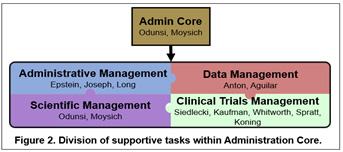
Biostatistics and Bioinformatics Core
Core Directors:
Alan Hutson, PhD
Song Liu, PhD
Theodore Karrison, PhD
The Biostatistics and Bioinformatics Core of the Roswell Park-University of Chicago Ovarian Cancer SPORE will assist basic, translational and clinical researchers of the SPORE with proper formulation, refinement and execution of study objectives by applying the appropriate biostatistics and bioinformatics analyses, and providing the appropriate interpretation of their results, in terms of both strengths and limitations. We will ensure a priori that Developmental Research Program and Career Enhancement Program projects are feasible from a biostatistical and bioinformatics perspective. The Biostatistics and Bioinformatics Core will ensure that appropriate data are collected; the data are of the highest quality and meet regulatory standards for privacy; and the data are those required to answer a study question, necessary for administrative reporting, needed to establish protocol compliance, and/or will be included in a manuscript. We will prepare Data Safety Monitoring Board reports and post clinical trial results to clinicaltrials.gov in a timely and compliant manner and ensure compliance to NIH policy and assist with data sharing of SPORE data via the NCI Cancer Research Data Commons infrastructure and similar NIH-designated data repositories.
Biospecimen and Pathology Core
Core Directors:
Carl Morrison, MD, DVM
Mark Lingen, DDS, PhD
The Roswell Park-University of Chicago Ovarian Cancer SPORE Biospecimen & Pathology Core (BPC) combines a centralized mechanism for expert tissue handling services and performance of tissue-based assays by highly qualified, experienced personnel with state-of-the-art integrated informatics infrastructure which provides tools for tracking biospecimen collection, histopathological evaluation, and clinical annotation of samples with linkage to immunogenomic and other translational data generated by the Individual Research Projects proposed in this renewal application. The Core will collect, de-identify, process, bank, and distribute annotated, high-quality tumor and blood samples from patients participating in SPORE-sponsored clinical studies. In addition, the BPC will provide investigators with specialized technical and interpretative histopathology services including expertise in multiplexed immunohistochemistry (IHC) and imaging mass cytometry (IMC), tissue microarray construction, laser capture microdissection, digital imaging, and quantitative pathology review of slides, tumor-infiltrating lymphocytes assessment, tissue QC/QA and preparation for next-gen sequencing-based assays (whole exome seq, RNAseq, TCRseq, MethylSEQ), and oversight on optimal utilization of each biospecimen. The Core will also be instrumental in testing patient biospecimens to determine eligibility for clinical trials and directly add to the bi-directional translational science by a detailed immunological annotation of ovarian tumors.
Immunogenomics Core
Core Directors:
Junko Matsuzaki, PhD
Per Basse, MD, PhD, DM.Sci.
The objective of the Immunogenomics Core is to perform comprehensive immunophenotyping, functional analyses, and genetic and epigenetic monitoring in peripheral blood (PB) and the tumor microenvironment (TME) and investigate tumor antigen-specific immune responses for the immunotherapeutic approaches being developed and tested in the Individual Research Projects (IRP) of the Roswell Park-University of Chicago Ovarian Cancer SPORE. It will be also responsible for immunologic and genomic assays for Career Enhancement Program (CEP) and Developmental Research Program (DRP).
The specific aims of the Immunogenomics Core are to:
- Design and optimize immunologic and genomic assays for IRPs, CEP and DRP, and provide state-of-the-art immunophenotyping, transcriptome analysis, TCR sequencing, epigenetic and functional analyses of immune cells in PB and TME treated with immunotherapy.
- Run whole-exome sequencing for tumor and PB samples to identify mutations and HLA types and confirm T-cell reactivity against the predicted neoantigens for DC-peptide vaccine in IRP2.
The Immunogenomics Core will develop new methodologies/markers and make them available to SPORE investigators. It also has a significant educational role, working with all SPORE PIs, technicians and young investigators on assay development, proper use of instrumentation, and interpretation of their data.
Developmental Research Program
Program Directors:
Kirsten Moysich, PhD, MS
Richard Koya, MD, PhD
The Developmental Research Program (DRP) of the Roswell Park-University of Chicago Ovarian Cancer SPORE is a platform to support innovative, potentially high-risk/high-reward ideas with the potential to mature further into translational ovarian cancer research projects that will transition basic and/or population research findings into research focused on patients or human specimens. DRP funding encourages the development of the pilot projects into collaborative, multi-investigator, multi-institutional translational research programs, or as new Individual Research Projects. DRP projects are the ultimate measure of the success of a SPORE, forming the basis for future translational ovarian cancer research and providing springboards for the next generation of R01, P01, Consortium, and SPORE grants. The goals of the proposal review process are not only to identify the most exciting projects, but to provide constructive critiques that are of value both for future submission of the projects to extramural funding sources, and for identifying potential collaborators and resources within the Roswell Park-University of Chicago Ovarian Cancer SPORE to increase the competitiveness of future applications. The DRP’s strength lies in its ability to respond rapidly to new initiatives and provide significant, timely financial support to high-risk/pilot projects.
Career Enhancement Program
Program Directors:
Kirsten Moysich, PhD, MS
Scott Abrams, PhD
Eileen Dolan, PhD
The purpose of the Career Enhancement Program (CEP) of the Roswell Park-University of Chicago Ovarian Cancer SPORE is to support the career development of junior researchers in translational ovarian cancer research. The target population for the program is outstanding entry-level (i.e. Assistant Professor) scientific and clinical faculty, but in some cases may include senior postdoctoral or clinical fellows with exceptional potential for independent research careers. A secondary goal of the CEP is to promote the diversity of ovarian cancer researchers, by encouraging the recruitment of outstanding women, underrepresented minorities and individuals with disabilities. The CEP will be supported by both SPORE funds and matching institutional funds from Roswell Park, the University of Chicago, and the University of Rochester. The CEP serves as a structured mechanism for identifying outstanding candidates through an open and competitive application and review process and matching the resulting awardees with experienced mentors. CEP awardees are afforded full access to the Roswell Park-University of Chicago Ovarian Cancer SPORE Core resources, including tissue specimens and statistical support. The CEP provides a vital mechanism for the development of the next generation of translational ovarian cancer researchers.
Institutional SPORE Website
https://www.roswellpark.org/research/roswell-park-uchicago-ovarian-cancer-spore







