University of Pittsburgh Head and Neck Cancer SPORE
University of Pittsburgh
Principal Investigators:

Robert L. Ferris, MD, PhD

Heath Skinner MD, PhD
- Principal Investigators Contact Information
- Overview
- Project 1: Hypoxia and metabolic dysregulation as a targetable barrier to immunotherapy in HNSCC
- Project 2: Optimizing patient selection and deintensified therapy for human HPV+ OPC
- Project 3: Determining biomarkers for responsiveness to immunotherapy targeting LAG3/PD-1 in HNSCC
- Administrative Core
- Tissue/Histology/Imaging Core
- Bioinformatics/Biostatistics Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigators Contact Information
Robert L. Ferris, MD, PhD
Director, UPMC Hillman Cancer Center
University of Pittsburgh
5150 Centre Ave, Suite 500
Fifth Floor Cancer Pavilion
Pittsburgh, PA 15232
Tel: 412-623-3205
Heath Skinner MD, PhD
Chair and Associate Professor, Department of Radiation Oncology
University of Pittsburgh
5117 Centre Avenue
Pittsburgh, PA 15232
Tel: 412-623-4047
Overview
The renewed SPORE from the University of Pittsburgh includes three projects to address three outstanding clinical questions in head and neck squamous cell carcinoma (HNSCC):
- Why does immunotherapy fail to benefit most HNSCC patients?
- What is the best strategy to decrease side effects using personalized treatment in human papillomavirus-positive (HPV+) HNSCC?
- What is the most rational way for integrating combination immunotherapy into currently available treatments?
To answer these questions, the SPORE provides critical infrastructure and resources, including an organ-specific database with detailed clinical and pathological information on over 12,000 patients followed for more than 30 years, four novel investigator-initiated clinical trials, and a team comprising both highly experienced and established investigators and accomplished early-stage investigators. Also included as part of the facilitating infrastructure are three SPORE-specific cores: (1) an Administrative Core to provide scientific and administrative oversight, support planning and evaluation, and oversee the organ specific database; (2) a Tissue/Pathology/Imaging Core to collect, characterize, and disperse biospecimens and clinical images; and (3) a Bioinformatics/Biostatistics Core to design and analyze sufficiently powered preclinical and clinical studies and integrate study results, including complex multi-omics data, into meaningful models. To ensure a continuous pipeline of qualified investigators and projects for advancing head and neck cancer research, the SPORE also includes a Career Enhancement Program and Developmental Research Program.
Project 1: Hypoxia and metabolic dysregulation as a targetable barrier to immunotherapy in HNSCC
Project Co-Leaders:
Greg M. Delgoffe, PhD (Basic Co-Leader)
Dan P. Zandberg, MD (Clinical Co-Leader)
This SPORE project is a continuation of Project 1 from the previous cycle of the SPORE grant, which evolved from a successful Developmental Research Program project. It is testing the hypothesis that tumor hypoxia represses T cell function in HNSCC and that the mechanism leading to this repression includes targetable markers of anti-PD-1 resistance. The project builds on published data showing that the metabolic landscape of the tumor microenvironment (TME) has a profound effect on patient response to checkpoint blockade immunotherapy and that inhibitors of oxidative phosphorylation can reduce hypoxia and improve anti-PD-1 responses in animal models. The three aims of Project 1 are shown in the accompanying schematic. This project will leverage biospecimens and computed tomography (CT) images made available by the Tissue/Pathology/Imaging Core and include two investigator-initiated trials conducted in patients with recurrent or metastatic HNSCC who are either naïve to anti-PD-1 immunotherapy (NCT04114136) or have progressed while receiving anti-PD-1 immunotherapy (NCT04326257). The trial in immunotherapy naïve patients is determining the effects of combining anti-PD-1 monotherapy with metabolism-altering drugs used to treat type 2 diabetes. It is comparing anti-PD-1 monotherapy with nivolumab to nivolumab + metformin and to nivolumab + rosiglitazone. The trial in anti-PD-1 resistant patients is determining whether addition of a second immunotherapy agent, which targets a different inhibitory receptor, can overcome innate or acquired anti-PD1 resistance. It is comparing the combination of nivolumab plus ipilimumab (anti-CTLA-4 agent) to nivolumab plus relatlimab (anti-LAG-3 agent).
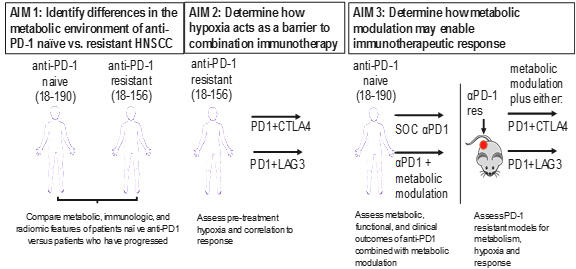
Description of project aims.
The preclinical and translational studies of Project 1 have been designed to answer the following questions pertaining to anti-PD1 immunotherapy resistance:
- What is the relationship between anti-PD-1 resistance, tumor metabolism, and hypoxia in HNSCC?
- Does hypoxia promote resistance to combinatorial immunotherapy in HNSCC?
- Can metabolically targeted therapy be combined with immunotherapy to overcome anti-PD1 resistance in HNSCC?
Successful completion of Project 1 will provide a predictive biomarker for assessing whether a patient is resistant to anti-PD-1 immunotherapy, which is now standard of care for advanced HNSCC, and may lead to larger clinical trials of combinatorial therapy.
Project 2: Optimizing patient selection and deintensified therapy for human HPV+ OPC
Project Co-Leaders:
Robert L. Ferris, MD, PhD (Clinical Co-Leader)
Heath D. Skinner, MD, PhD (Basic Co-Leader)
This new SPORE project builds on the completed phase II ECOG E3311 clinical trial in patients with HPV+ HNSCC of the oropharynx (NCT01898494), which evaluated minimally invasive trans-oral robotic surgery (TORS) by credentialed surgeons, followed by determination of recurrence risk (based on tumor and lymph node pathology) and risk-adapted adjuvant therapy. Although the goal of that trial was to reduce treatment-induced disfigurement and toxicity without negatively impacting survival, approximately 30% of the patients were determined to have high-risk neck disease (extranodal extension(s) greater than 1 mm and/or five or more lymph nodes harboring cancer) and thus received an escalated radiation dose along with cisplatin chemotherapy.
Project 2 is designed to verify whether a four-gene mutational and/or radiomic risk signature, which was identified in preliminary studies on a subset of ECOG E3311 patients, can be used or optimized to differentiate which high-risk neck disease patients can safely receive a lower radiation dose following TORS. The project is utilizing biospecimens, CT images, and clinical data from a larger group of E3311 patients and from a separate cohort of standard-care UPMC Hillman Cancer Center patients. The tumor samples are also being used to define the relationship between mutations, immune infiltrate, and repression of interferon signaling.
To obtain a prospective cohort for validation and generalization of the findings from ECOG E3311 patients, an ongoing investigator-initiated clinical trial in HPV+ HNSCC patients who are found to have high-risk neck disease after TORS (NCT03715946) has been modified. In addition to the original trial design in which patients were randomized to receive adjuvant anti-PD-1 immunotherapy + standard or dose-deintensified radiotherapy, additional arms were added in which patients receive adjuvant cisplatin chemotherapy + standard or dose-deintensified radiotherapy, to match the high risk neck disease arm in the E3311 trial.
Successful completion of Project 2 will produce a validated and immediately applicable predictive signature for patients with high-risk neck disease and an explanation for the repressed immune infiltrate seen in those patients. It will also assess the extent to which quality of life and toxicity are improved in the high-risk patients who receive TORS plus reduced radiation doses.
Project 3: Determining biomarkers for responsiveness to immunotherapy targeting LAG3/PD-1 in HNSCC
Project Co-Leaders:
Tullia Bruno, PhD (Basic Co-Leader)
Dario Vignali, PhD (Basic Co-Leader)
Dan P. Zandberg, MD (Clinical Co-Leader)
This new SPORE project evolved from a successful Developmental Research Program project funded during the prior SPORE grant cycle and leverages a recent clinical trial of combinatorial immunotherapy (NCT01968109) that included patients with HNSCC. It is addressing the overarching hypothesis that LAG3/PD1 dual blockade synergizes to promote CD8+ T cell function in the tumor and peripheral blood of HNSCC patients, and that a LAG3-dominant inhibitory receptor module in peripheral CD8+ T cells downregulates T cell function and ultimately patient response to PD1-targeted therapy. The two aims of Project 3 are shown in the accompanying schematic. The project includes a new SPORE-specific neoadjuvant window trial in which treatment-naïve locally advanced HNSCC patients are being randomized to receive either nivolumab (anti-PD-1) monotherapy or the combination of nivolumab + relatlimab (anti-LAG-3) followed by surgery, and utilizes new single-cell, multi-parametric technologies made available by the Tissue/Histology/Imaging Core to characterize immune cell populations in the TME and periphery. The project’s studies have been designed to address two key questions:
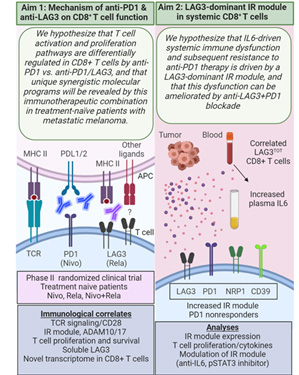
Description of project aims.
- What is the mechanism of anti-PD-1 and anti-LAG3 on the anti-tumor function of CD8+ T cells?
- Does IL6 drive a LAG3-dominant IR module in peripheral CD8+ T cells and does it predict responsiveness to immunotherapy?
Successful completion of Project 3 will define biomarkers of response and resistance to nivolumab and nivolumab + relatlimab and identify a patient population that will optimally benefit from inclusion of LAG3-based therapies.
Administrative Core
Core Directors:
Robert L. Ferris, MD, PhD
Heath D. Skinner, MD, PhD
Dan P. Zandberg, MD
The Administrative Core provides scientific leadership and general administration for all SPORE-related activities to integrate diverse scientific and clinical disciplines into a truly multidisciplinary team with the aims of advancing HNSCC translational research and improving the lives of HNSCC patients. This multidisciplinary approach is reflected in the Core’s leadership, which comprises a surgeon/scientist (Dr. Ferris), a radiation oncologist/scientist (Dr. Skinner), and a medical oncologist/clinical trialist (Dr. Zandberg), who share responsibility for directing and managing the SPORE.
The Administrative Core coordinates all SPORE Projects, Cores, and Programs, with an emphasis on maximizing collaborative interactions and efficiency. It oversees day-to-day operations with the assistance of a SPORE Administrator (Ms. Platania); coordinates administrative and scientific reviews by the SPORE’s executive committee, patient advocates, and internal and external advisory boards; and works closely with NCI staff and other SPORE teams to further NCI Translational Research Program goals. The Administrative Core is responsible for managing SPORE resources and ensuring compliance with all regulatory requirements. It maintains the Organ Specific Database, which, in addition to being updated with entries for new head and neck cancer patients and SPORE studies, is being expanded to include quality of life data, which are of particular importance to patients whose ability to speak, swallow, and recognize themselves in the mirror can be impacted by HNSCC and its treatment.
Tissue/Histology/Imaging Core
Core Directors:
Simion I. Chiosea, MD
Rivka Colen, MD
Raja Seethala, MD
The Tissue/Histology/Imaging Core is a central repository of blood, tissue, and imaging resources for all investigators in the SPORE and collaborators within and outside of the institution. In all, the Core provides access to banked biospecimens from more than 12,000 patients. Building upon 16 years of experience, the Core facilitates prospective specimen collection, including blood and tissue from primary and recurrent HNSCC under an IRB-approved protocol that has been in place since 1999, and performs sample preparation, immunohistochemistry, in situ hybridization, and whole exome sequencing. To support the projects in the current SPORE renewal cycle, the Core has incorporated additional technologies, including bulk and single cell RNA sequencing, multi-parametric flow cytometry and multi-spectral imaging, with newly acquired state-of-the-art instrumentation, including Aurora Cytek, CODEX, and Vectra Polaris. Also new to the Core is integration of patient standard-of-care CT images and radiomics into a seamless workflow. The Core leverages the expertise, instrumentation, and services of UPMC Hillman Cancer Center’s Tissue and Research Pathology Services, Cancer Genomics Facility, Cytometry Facility, Immunologic Monitoring and Cellular Products Laboratory, and In Vivo Imaging Facility, which are vetted shared resources that receive Cancer Center Support Grant funding from the NCI.
Bioinformatics/Biostatistics Core
Core Directors:
Riyue Bao, PhD
Hong Wang, PhD
The Biostatistics/Bioinformatics Core supports the experimental design, analysis, visualization, integration, and reporting needs of all SPORE, developmental research, and career enhancement projects. This includes projects that generate multiple types of high-dimensional data, including bulk and single-cell genomic, immunologic, and multiplex immunohistochemistry data from patient and animal specimens. The Core leverages the expertise, analytical tools, and data processing and storage capabilities of UPMC Hillman Cancer Center’s Cancer Bioinformatics Services and Biostatistics Facility, which are vetted shared resources that receive Cancer Center Support Grant funding from the NCI. As required, the Biostatistics/Bioinformatics Core develops new analytic strategies, working closely with SPORE project investigators.
Developmental Research Program
Program Directors:
Umamaheswar Duvvuri, MD, PhD
Marci Lee Nilsen, PhD, RN
Heath D. Skinner, MD, PhD
By combining SPORE and institutional funds, the SPORE’s Developmental Research Program funds up to four projects per year. The program’s new two-tiered review process ensures that the projects have high potential for generating data to support a new SPORE or R01-equivalent project, are appropriately powered to achieve their stated aims, and are appropriately budgeted. Each of the three Career Enhancement Program Co-Directors represents a different discipline of importance to head and neck cancer research.
Career Enhancement Program
Program Directors:
Christopher Bakkenist, PhD
Tullia Bruno, PhD
Dan P. Zandberg, MD
By combining SPORE and institutional funds, the SPORE’s Career Enhancement Program funds up to two investigators per year. The program’s new two-tiered review process ensures that the projects qualify as translational, are scientifically feasible and appropriately powered to achieve their stated aims, include a qualified mentor, and are appropriately budgeted. Each of the three Career Enhancement Program Co-Directors represents a different career stage and each has personally received funding from a SPORE career enhancement and/or developmental research program.







