Mayo Clinic Hepatobiliary SPORE
Mayo Clinic
Principal Investigator(s):

Mark McNiven, PhD

Lewis Roberts, MB, ChB, PhD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Diagnostics and Drug Repurposing for Fibrolamellar Hepatocellular
- Project 2: Targeting YAP-TEAD Oncogenic Signaling and Therapeutic Resistance in Cholangiocarcinoma (CCA)
- Project 3: Reversal of the Immune Suppressive HCC Microenvironment Through Appropriately Timed and Optimally Delivered Combination Viroimmunotherapies
- Administrative Core
- Biostatistics and Bioinformatics Core
- Biospecimens and Pathology Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigator(s) Contact Information
Mark A. McNiven, PhD
George M. Eisenberg Professor of Biochemistry and Molecular Biology
200 First Street, SW
Guggenheim Building 1642D,
Rochester, Minnesota, 55905
(507) 284-0683
Lewis R. Roberts M.B., Ch.B., Ph.D.
Peter and Frances Georgeson Professor of Gastroenterology Cancer Research
Mayo Clinic
200 First Street SW Mayo Building, 9 West
Rochester, Minnesota, 55905
(507) 422-6542
Overview
The mission of the Mayo Clinic SPORE in Hepatobiliary Cancers (HBC) is to improve prevention, early detection, diagnosis, and treatment of cancers of the liver and biliary tree (hepatobiliary) through impactful team-based translational research. These cancers, namely hepatocellular carcinoma and cholangiocarcinoma, rank as the second leading cause of cancer-related death worldwide. The HBC SPORE will achieve its goals by combining talented basic and clinical scientists with state-of-the-art technologies and closely coordinated leadership to continue building a strong continuum of basic, clinical, and translational research. Mayo is well positioned to support this program given its strong history of interdisciplinary infrastructure (including several other current NCI-sponsored SPOREs) that closely links basic and clinical research to promote scientific collaboration. This team-based approach is a Mayo hallmark and a cornerstone of the SPORE’s organizational structure. Likewise, our leadership, both overall and for each project, incorporates a team approach that unites investigators with basic science and clinical/applied portfolios. This SPORE will continue to springboard from an established, solid research base, as it has in the first cycle of funding, with three new, promising translational proposals, each grounded upon talented Mayo and external investigators. The overall SPORE leadership is well equipped to continue guiding this HBC program forward, combining a patient-centered approach to clinical care, a rigorous research perspective, and an experienced hand in education and mentorship. The leadership and Administrative Core facilitate cross core communication and interaction with the translational research projects and pilot project programs while also stimulating scientific collaborations and expediting bi-directional community engagement of diverse patient populations. The proposed translational projects are paired with unique clinical trials to develop diagnostics and advance mechanistic understanding of novel therapies while also identifying the patients most likely to benefit.
Project 1: Diagnostics and Drug Repurposing for Fibrolamellar Hepatocellular Carcinoma
Project Co-Leaders:
Michael S. Torbenson, M.D. (MCR) (Applied/Clinical Co-Leader)
Sanford Simon, Ph.D. (RU) (Basic Science Co-Leader)
Fibrolamellar hepatocellular carcinoma (FLC) is an often-lethal liver cancer that affects mainly children and young adults. This cancer usually is diagnosed at an advanced stage because it has vague symptoms. The rate of recurrence is high, even for those who undergo surgical resection. The poor outcome is made even worse by the lack of existing diagnostic tests and no approved therapy. This research project is tackling these obstacles.
Specific Aims:
Aim 1: Develop the first diagnostic tests for FLC. The project is developing two kinds of tests: noninvasive blood tests and tests to provide a molecular diagnosis on biopsy samples.
Aim 2: Screen for compounds that can selectively kill FLC. As two of the world’s most prominent investigators of this specific cancer, Project 1 Co-Leaders discovered that patients with FLC have a single, consistent deletion in one copy of chromosome 19. This leads to a novel fusion of two genes, resulting in a chimeric gene encoding a fusion protein that is an active subunit of protein kinase A. They found that expression of the active fusion kinase is sufficient to initiate FLC in the livers of mice and targeting the active kinase fusion junction can eliminate FLC. Thus, FLC appears to be the result of a single change to which the tumor is addicted. The investigators are working to block the activity of the fusion kinase. The goal is to find an FDA-approved compound that selectively blocks the kinase activity of the FLC fusion protein and repurpose it as a novel therapy for this lethal liver cancer.
Project 2: Targeting YAP-TEAD Oncogenic Signaling and Therapeutic Resistance in Cholangiocarcinoma (CCA)
Project Co-Leaders:
Mitesh Borad, M.D. (MCA) (Applied/Clinical Co-Leader)
Rory Smoot, M.D. (MCR) (Applied/Clinical and Basic Science Co-Leader)
Gregory Gores, M.D. (MCR) (Basic Science Co-Leader)
Aim 1: We will evaluate whether sustained YAP signaling is required for cholangiocarcinoma tumors to develop and continue growing in a mouse model in which we can turn YAP on and off during tumor development and growth. To understand the therapeutic implications of this signaling we will define the effects of a novel YAP-TEAD inhibitor in multiple cholangiocarcinoma cell lines and models.
Aim 2: To test the hypothesis that YAP-TEAD signaling can alter the tumor immune microenvironment utilizing our inducible YAP knockout models. We will evaluate the immune infiltrate broadly but also with specific attention to myeloid-derived suppressor cells. To understand the therapeutic implications of this signaling we will define the effects of YAP-TEAD inhibition in combination with immune checkpoint inhibition utilizing humanized mice.
Aim 3: We will test TEAD inhibition as a therapeutic approach by performing an early phase clinical trial with a novel TEAD inhibitor. This phase 1 study will facilitate additional clinical trial phases.
Translational impact: The translational impact of these studies is in defining the role and mechanisms of YAP-TEAD signaling that support cholangiocarcinoma tumor development, growth, and resistance to therapy. These preclinical studies and clinical trial will provide crucial data for continuing development of TEAD targeting strategies in cholangiocarcinoma.
Project 3: Reversal of the Immune Suppressive HCC Microenvironment Through Appropriately Timed and Optimally Delivered Combination Viroimmunotherapies
Project Co-Leaders:
Mitesh Borad, M.D. (MCA) (Applied/Clinical Co-Leader)
Lewis R. Roberts, M.B., Ch.B., Ph.D. (MCR) (Applied/Clinical and Basic Science Co-Leader)
Richard G. Vile, Ph.D. (MCR) (Basic Science Co-Leader)
This project focuses on the development of genetically modified tumor-killing viruses, including the vesicular stomatitis virus (VSV), to treat hepatocellular carcinoma (HCC), or liver cancer. The investigators are combining these viruses with clinically used immunotherapy treatments (immune checkpoint inhibitors) to leverage enhanced killing of the tumor by immune T cells. Additionally, they are engineering new viruses that will further enhance the immune response, testing them in laboratory models and then advancing the most promising candidates into clinical trials. These new cancer killing viruses are engineered to express an immune stimulatory gene (interferon-β) in combination with cancer cell division checkpoint inhibitor antibodies in animal models and in an early-phase clinical trial. This next-generation tumor killing viral platform is custom engineered to the treatment of HCC and is injected directly into liver cancers. The project’s established animal models demonstrate that the combination of a systemic checkpoint inhibitor together with an intratumorally delivered tumor killing virus can significantly improve survival outcomes over either treatment alone and that survival benefit is associated with a striking improvement in anti-tumor memory responses. The investigative team hypothesize that the tumor killing VSV provides a complementary mechanism of action to immune checkpoint inhibition and that this combination can be used to effectively treat HCC.
Specific Aims:
Aim 1: Determine the optimal dosing regimens and timing of combination checkpoint blockade therapy with engineered tumor killing VSV in laboratory models of HCC.
Aim 2: To combine engineered specialized immune cells (CAR T cells) with VSV virotherapy to enhance standard of care immunotherapy in mouse and human tumor models of HCC.
Aim 3: To test the safety of engineered next-generation VSV virotherapy in a Phase I clinical study as therapy in patients with advanced HCC who have already received standard of care treatment.
Administrative Core
Core Directors:
Mark A. McNiven, Ph.D. (MCR) (Director)
Lewis R. Roberts, M.B., Ch.B., Ph.D. (MCR) (Co-Director)
The purpose of the Administrative Core is to provide the organizational infrastructure necessary to promote innovative and collaborative research in hepatobiliary cancer (HBC) and to expedite the translation of discoveries into more effective methods of prevention, detection, and treatment of these lethal cancers. The Core continues to provide an organizational structure that coordinates the activities of translational research projects, scientific cores, community outreach, Developmental Research Program, and Career Enhancement Program at all three Mayo campuses in Rochester, MN (MCR), Jacksonville, FL (MCF), and Phoenix/Scottsdale, AZ (MCA) and affiliated institutions (NCI, Rockefeller University, University of Pittsburgh). The Core coordinates the advisory functions of the Internal and External Advisory Boards, Patient Advocates, and the Community Engagement and Diversity Council. The HBC SPORE leadership brings together unique and complementary expertise with synergistic efforts as Co-Directors of this Core.
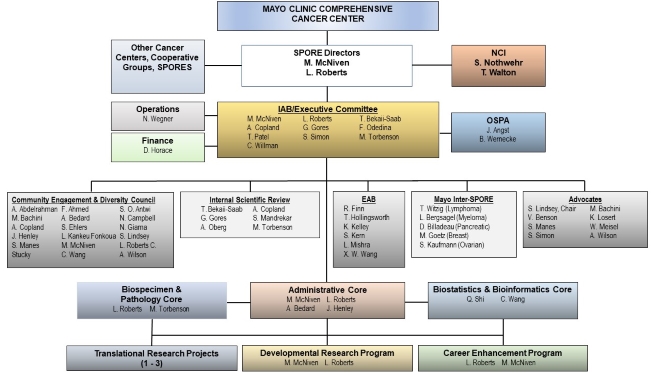
Organizational Structure of the Mayo Clinic HBC SPORE with Oversight Provided by the Administrative Core.
Biostatistics and Bioinformatics Core
Core Directors:
Qian Shi, Ph.D. (MCR) (Co-Director)
Chen Wang, Ph.D. (MCR) (Co-Director)
The Biostatistics and Bioinformatics Core provides statistical, bioinformatics, computational biology collaboration, and data management support for the Hepatobiliary Cancer SPORE translational research projects and cores. The core also provides data management for clinical trials, monitors adverse events, and prepares data summaries for manuscript preparation. The Biostatistics and Bioinformatics Core works closely with the Hepatobiliary Cancer SPORE's other cores to ensure a smooth continuum of data flow for clinical trials.
Specific Aims:
Aim 1: Provide SPORE investigators access to expertise in collaborative development of study designs and analysis plans, state-of-the-art data analysis and interpretation, data management resources, sequencing alignment, variant calling, data analysis and quality assurance, pathway analysis, and access to and analysis of data in public databases.
Aim 2: Provide management and integration of existing and newly collected data through consistent and compatible data handling.
Aim 3: Support the Biospecimen and Pathology Core by providing resources to the research community and a centralized system to facilitate collaboration and data reuse.
Biospecimens and Pathology Core
Core Directors:
Michael S. Torbenson, M.D. (MCR) (Director)
Lewis R. Roberts, M.B., Ch.B., Ph.D. (MCR) (Co-Director)
This core provides investigators and collaborators in the Hepatobiliary Cancer SPORE with access to high-quality deidentified patient data, DNA, plasma, serum, and cancer tissue biospecimens from patients with hepatobiliary cancer. The core continues to build upon the International Hepatobiliary Neoplasia Biorepository within the Hepatobiliary Cancer SPORE. Additionally, the core coordinates with the Fibrolamellar Hepatocellular Carcinoma Biorepository led by Project 1 Leader Sandy Simon, Ph.D. at Rockefeller University (NY). The core is supported by the Mayo Clinic Comprehensive Cancer Center: specifically, 1) by the Biospecimens Accessioning and Processing Core for processing blood to genomic DNA, serum, and plasma samples, and 2) by the Pathology Research Core for histology and other tissue-based services, including paraffin and frozen sectioning, immunohistochemistry, tissue microarray construction, and digital imaging.
Specific Aims:
Aim 1: Obtain patient informed consent for blood and tissue specimens.
Aim 2: Abstract patient clinical information into a secure web-based registry that includes demographics, tumor stage, treatments, and clinical outcomes.
Aim 3: Provide clinically annotated blood and tissue specimens for clinical and translational research within the SPORE.
Aim 4: Provide clinically annotated patient-derived cell lines, organoids, tumor xenografts, plus animal models, including expertise in Sleeping Beauty transposon-induced liver cancer models.
Developmental Research Program
Program Directors:
Mark A. McNiven, Ph.D. (MCR) (Director)
Lewis R. Roberts, M.B., Ch.B., Ph.D. (MCR) (Co-Director)
The goal of the Developmental Research Program (DRP) of the NCI-supported Mayo Clinic SPORE in Hepatobiliary Cancers is to support innovative, scientifically sound research projects from which findings can be translated into clinically relevant interventions that reduce the burden of liver and biliary cancers, which are a leading cause of cancer related deaths that continues to grow. The DRP provides up to $50k per year to each of 2 projects per year, with the potential of a second year of support based on progress and promise. Potential high risk/high payoff innovative concepts are encouraged that utilize the extensive shared resources cores of the SPORE. Investigators from a broad array of demographic groups are encouraged to apply. A rigorous peer review process utilizing the expertise of the External Advisory Board and other experienced investigators, with additional input from the patient advocates and Community Engagement and Diversity Council, evaluates all applications based upon the following criteria: scientific merit, originality, qualifications of the applicant/co-investigator(s), and translational potential. The long-term goal of the SPORE is to translate the findings generated by these developmental projects into a reduction in the incidence and mortality rates of liver and biliary cancers.
Career Enhancement Program
Program Directors:
Lewis R. Roberts, M.B., Ch.B., Ph.D. (MCR) (Director)
Mark A. McNiven, Ph.D. (MCR) (Co-Director)
Fundamental to advancing prevention, diagnosis, and treatment of hepatobiliary cancer (HBC) is the engagement and focus of dedicated researchers. The central activities of the Career Enhancement Program (CEP) meet this fundamental need by identifying, developing, and monitoring the progress of the most promising investigators for translational research in liver and biliary cancer. The CEP has demonstrated a strong track record of supporting applicants from two targeted groups: 1) early-stage investigators and 2) mid-career investigators who previously have not engaged in HBC research. The support includes financial ($100k/year) and extensive mentorship. Qualified investigators from a broad range of demographic groups are encouraged to apply. This comprehensive program ultimately will create an interdisciplinary network of researchers committed to the prevention, diagnosis, and treatment of HBC. Awards support projects for 1 year, with a requirement that adequate need or progress is demonstrated for a second year of funding. The expectation is that successful CEP proposals will replace full SPORE projects or be eligible for other sources of extramural funding. The CEP is a key component of the Mayo Clinic SPORE in HBC, providing a significant mechanism for increasing the number of qualified scientists and clinical investigators who are committed to translational HBC research.
Institutional SPORE Website
Mayo Clinic Hepatobiliary SPORE
(https://www.mayo.edu/research/centers-programs/cancer-research/research-programs/gastrointestinal-cancer-program/mayo-clinic-hepatobiliary-spore)







