Dana-Farber/Harvard Cancer Center SPORE in Lung Cancer
Dana-Farber Cancer Institute
Principal Investigator(s):

David Barbie, MD

Lecia Sequist, MD, MPH
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Peptide vaccination against ALK in the treatment of patients with tyrosine kinase inhibitor-resistant ALK-positive lung cancer
- Project 2: Targeting replication stress in SMARCA4-mutant NSCLC
- Project 3: Targeting minimal residual disease in EGFR-mutant lung cancer
- Administrative Core
- Pathology and Genomics Core
- Biostatistics Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigator(s) Contact Information
David Barbie, MD
Associate Professor of Medicine at Harvard Medical School and the Lowe Center for Thoracic Oncology
Associate Director of the Robert and Renée Belfer Center for Applied Cancer Science
Dana-Farber Cancer Institute
450 Brookline Ave, LC4118C
Boston, MA 02215
Tel: 617-632-6036
Lecia Sequist, MD, MPH
Landry Family Professor of Medicine, Harvard Medical School
Director, Center for Innovation in Early Cancer Detection
Massachusetts General Hospital
55 Fruit Street
Boston, MA 02114
Tel: 617-726-7812
Overview
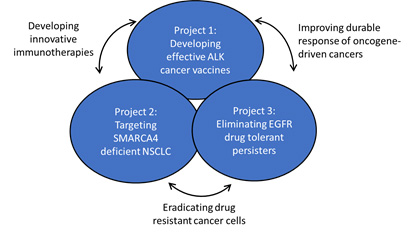
The Dana-Farber/Harvard Cancer Center (DF/HCC) Lung SPORE seeks to capitalize on important gains in targeted and immunotherapy of lung cancer and develop innovative translational strategies designed to improve lung cancer cure rates.
Description of projects.
The overarching goals of this SPORE are:
Aim 1: Design immunologic therapies that harness both the innate and adaptive immune systems to overcome ALK inhibitor resistance and enhance efficacy of PD-1 immune checkpoint blockade in non-small cell lung cancer (NSCLC).
Aim 2:Develop innovative approaches to EGFR and ALK-driven lung cancer with potential to improve long term survival via cancer vaccines or elimination of drug tolerant persister (DTP) cells or cancer vaccines.
Aim 3: Co-opt vulnerabilities such as replication stress in SMARCA4 mutant NSCLC or a senescence program in EGFR TKI DTPs.
Aim 4: Foster inter-institutional collaboration, including exchange of lung cancer models and patient samples.
Aim 5: Continue to support and develop the next generation of lung cancer translational scientists from our talented group of fellows and early career investigators.
Project 1: Peptide vaccination against ALK in the treatment of patients with tyrosine kinase inhibitor-resistant ALK-positive lung cancer
Project Co-Leaders:
Mark Awad, MD (Clinical Co-Leader)
Roberto Chiarle, MD (Basic Co-Leader)
Justin F. Gainor, MD (Clinical Co-Leader)
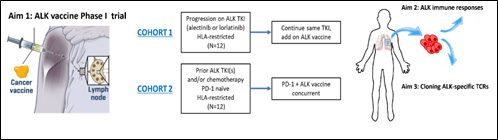
The goal of Project 1 is to develop a therapeutic ALK vaccine for advanced ALK-rearranged (ALK+) NSCLC. Acquired resistance to all available FDA-approved ALK TKIs is inevitable, at which time patients are typically treated with cytotoxic chemotherapy, which has only modest efficacy. Similarly, ICIs have little activity in ALK+ NSCLC, leaving no effective approaches for treating TKI-resistant disease. Since ALK is uniquely expressed in lung cancer cells, it is an ideal tumor associated antigen vulnerable to immunotherapeutic strategies that strive to achieve our overall goal of improving survival for patients with ALK+ lung cancers. This Project builds on an unparalleled foundation of excellence in the field of ALK+ lung cancer at DF/HCC. Given our unique expertise we have become major referral centers for ALK+ patients.
Description of Project 1.
Project 2: Targeting replication stress in SMARCA4-mutant NSCLC
Project Co-Leaders:
David Barbie, MD (Basic Co-Leader)
Ibiayi Dagogo-Jack, MD (Clinical Co-Leader)
Carla Kim, PhD (Basic Co-Leader)
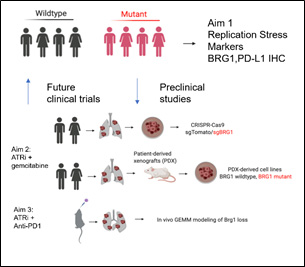
The purpose of Project 2 is to develop novel treatment approaches for an emerging and clinically relevant subtype of NSCLC that currently lacks effective therapeutic options. SMARCA4 is one of the most frequently mutated genes in NSCLC, present in 9- 11% of patients, inactivating its gene product BRG1, a key subunit of the SWI/SNF chromatin remodeling complex. SMARCA4 mutations often overlap with molecular alterations (KEAP1, STK11) that decrease sensitivity to immunotherapy and chemotherapy; indeed, in retrospective series from MGH and MSKCC, NSCLC SMARCA4 mutations in advanced NSCLC were associated with poor prognosis and unfavorable disease characteristics. Thus, effective targeting of SMARCA4 NSCLC is a key unmet need.
Description of Project 2.
Project 3: Targeting minimal residual disease in EGFR-mutant lung cancer
Project Co-Leaders:
Aaron Hata, MD, PhD (Basic Co-Leader)
Pasi Janne, MD, PhD (Basic Co-Leader)
Lecia Sequist, MD, MPH (Clinical Co-Leader)
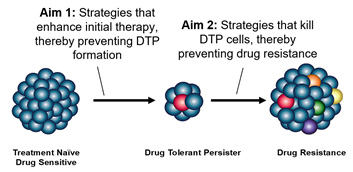
The overall goal of Project 3 is to enhance the durable activity of targeted therapies for lung cancer. The best and most deeply characterized subset for this is advanced EGFR mutant lung cancer, which, despite all prior success, remains an incurable disease. Work from our investigators and others has solidified the fact that after an initial treatment response, tumors enter a quiescent phase (drug tolerant persister (DTP) state) lasting months or even years before tumor regrowth and acquired drug resistance occurs. Most strategies to enhance genotype directed therapy have focused on targeting molecular changes at the time of resistance or intensifying initial treatment approaches. But they fail to address the specific mechanisms of the DTP state, which could be targeted earlier to eradicate these and prevent resistance.
Description of Project 3.
Administrative Core
Core Directors:
David Barbie, MD
Lecia Sequist, MD, MPH
The goal of the proposed Administration Core is to facilitate the functions and management of the Lung SPORE and to ensure the research is performed with rigor and to the highest possible standards.
Pathology and Genomics Core
Core Directors:
John Iafrate, MD, PhD
Neal Lindeman, MD
The Integrated Pathology and Genomics Core provides state-of-the-art analytic services for lung cancer samples spanning the full spectrum from initial sample handling and processing to microscopic analysis by certified anatomic pathologists, to immunophenotypic analysis, to molecular genetic and genomic analysis. This work constitutes the backbone of lung cancer management and enables the diagnosis and proper treatment selection for patients with lung cancers of all types, and also enables research into cancer biology, etiology, and management.
Biostatistics Core
Core Director:
Beow Yeap, ScD (Director)
During the last few decades, the development of novel and powerful statistical tools as well as increasing access to low-cost, high-speed computing have made significant impacts on cancer research. Consequently, an expanded and critical role has emerged for statisticians to collaborate in the investigative process, and more importantly, a higher and more rigorous standard has been established and accepted widely in the definition of scientific evidence. Notably, the rapid advances in genetics and genomics through large-scale molecular approaches to personalized medicine have presented new challenges in experimental design and data analysis. The Biostatistics Core investigators have been chosen for their complementary scope of biostatistical expertise and research experience in animal models and clinical trials as well as correlative analyses of clinical, pathologic, and molecular data.
Developmental Research Program
Program Directors:
Rebecca Heist, MD, MPH
David Kwiatkowski, MD, PhD
Henning Willers, MD
DRP awards are for investigators at any stage of their career with an idea for an innovative, high-risk project related to lung cancer. A funded DRP project may provide preliminary data that synergizes with the overarching objectives of the SPORE and could subsequently lead directly to a new SPORE project. DRP awards open the door to bring new investigators into the SPORE program, catalyzing and facilitating interest in lung cancer research among a diverse range of scientists. The DRP also provides an opportunity for Dana-Farber/Harvard Cancer Center (DF/HCC) investigators to collaborate with SPORE and non-SPORE investigators at other cancer centers and/or around the world.
Career Enhancement Program
Program Directors:
Daniel Costa, MD, PhD
Bruce Johnson, MD
Deepa Rangachari, MD
CEP awards provide SPORE leadership with the ability to fund new proposals and promising young investigators each year, infusing the team with potential new directions. CEP awards focus on supporting and mentoring junior investigators in their initial pursuit of a career in translational lung cancer research. Indeed, an ever-increasing pressure is placed upon young investigators to become productive and self-supporting early in their careers; however, translational research, by its very nature, often requires multiple years to achieve meaningful patient endpoints. The CEP is a mechanism to protect the time of clinically applied and biologically applied scientists, enabling them to develop into independent translational scientists.







