City of Hope Leukemia SPORE
City of Hope
Principal Investigator(s):

Stephen J. Forman, MD

Larry W. Kwak, MD, PhD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: CMV/CD19 bi-Specific CAR T cells combined with CMV vaccine as post-transplantation immunotherapy for non-Hodgkin lymphoma
- Project 2: Therapy-related leukemia following autologous transplantation for lymphoma
- Project 3: Targeted immunotherapy for relapsed/refractory Hodgkin lymphoma
- Project 4: Novel nucleotide-based approaches targeting the STAT3 pathway for the treatment of lymphoma
- Administrative Core
- Biostatistics and Bioinformatics Core
- Tissue Bank Core
- Manufacturing Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigator(s) Contact Information
Stephen J. Forman, MD
Director, T Cell Therapeutics Research Laboratories
Director, Hematologic Malignancies Research Institute
Professor, Department of Hematology & Hematopoietic Cell Transplantation
Institution
City of Hope
1500 East Duarte Road
Duarte, CA 91010
Tel: (626) 218-2405
Larry W. Kwak, MD, PhD
Deputy Director, Hematologic Malignancies Research Institute
Director, Toni Stephenson Lymphoma Center
Dr. Michael Friedman Endowed Professor
City of Hope
1500 East Duarte Road
Duarte, CA 91010
Tel: (626) 218-8913
Overview
The overall goal of the City of Hope (COH) Lymphoma SPORE is to develop powerful new therapeutics based on basic and preclinical observations in molecular and cellular immunology at COH laboratories. In addition, 5 clinical trials are proposed, 4 of which utilize agents (cells, small molecules, radiolabeled antibodies) that are being produced at COH GMP facilities (Core D). In Project 1, we engineered T cells that respond to both lymphoma and cytomegalovirus (CMV) antigen. A CMV vaccine that was developed at COH will be used to boost T cell expansion persistence and allow in vivo control of CMV-CD19 CAR T cells as a post-transplant immunotherapy for non-Hodgkin lymphoma. In Project 2, we build on our previously published prospective longitudinal study of lymphoma patients undergoing autologous hematopoietic cell transplantation (autoHCT), and their susceptibility to therapy-related myeloid neoplasm (t-MN). COH patients from this longitudinal study now serve as the discovery cohort, along with an external multi-institutional cohort to validate a new risk-prediction model that incorporates clonal hematopoiesis of indeterminate potential (CHIP) and other patient clinical and genetic features to estimate the likelihood of developing t-MN after autoHCT. Project 3 addresses the poor outcomes for patients with relapsed Hodgkin lymphoma with two phase 2 clinical trials. A PET-adapted strategy using PD-1 inhibitor nivolumab ± ICE chemotherapy as a bridge to autoHCT has shown that many patients can to proceed to autoHCT without salvage chemotherapy. The aTac-BEAM trial (recently activated) is an anti-CD25 radioimmunotherapy-based augmented autoHCT regimen. Project 4 will utilize two unique agents produced by our own cGMP production facility to target the intracellular transcription factor STAT3 in patients with lymphoma. Specifically, we will test a modified oligonucleotide that silences STAT3 in a clinical trial (recently activated) in Specific Aim 1 (SA1), and in SA2 we will test a modified high-affinity STAT3-DNA binding sequence that effectively competes with STAT3 DNA binding. We have thus far funded 3 CEP and 4 DRP awards which include 2 women, 1 African and 3 Asian recipients. These awards have thus far yielded one NIH R01 and an R03 grant, 2 patent applications, and an IND submission.
Project 1: CMV/CD19 bi-Specific CAR T cells combined with CMV vaccine as post-transplantation immunotherapy for non-Hodgkin lymphoma
Project Co-Leaders:
Stephen J. Forman, MD (Clinical Science Co-Leader)
Ryotaro Nakamura, MD (Clinical Science Co-Leader)
Donald Diamond, PhD (Basic Science Co-Leader)
Xiuli Wang, PhD (Basic Science Co-Leader)
We propose selecting cytomegalovirus (CMV) pp65-specific T cells for ex vivo modification with our phase-1-tested CD19 CAR, infusing the CMV-CD19 bi-specific T cells into patients after hematopoietic cell transplantation (HCT), and then expanding in vivo via CMV-specific modified vaccinia Ankara (MVA) Triplex vaccine injections (Figure 1). This strategy is feasible since more than 70% of adults are CMV immune, the frequency of pp65-specific T cells in CMV+ donors is high, and there is clinical experience with adoptive cellular immunotherapy targeting the CMVpp65 antigen. Importantly, in the post-allogeneic HCT setting, use of CMV selection has a low risk of inducing GVHD and the possible benefit of preventing CMV infection. City of Hope researchers have also developed a novel CMV vaccine, Triplex, which can safely stimulate CMV-specific T cell immunity, through endogenous T cell receptors (TCR). This vaccine will be administered to patients after infusion of CMV-CD19CAR T cells to provide an antigen boost to trigger in vivo expansion of the CMV-specific CAR T cells. Triplex is a multi-antigen recombinant MVA virus vaccine that has thus far proven safe and immunogenic in a phase 1 trial in healthy volunteers, and safe and effective at preventing CMV reactivation in a phase 2 vaccine trial in allo HCT recipients (NCT02506933).
Hypothesis: CD19CAR-engineered CMVpp65-specific T cells are capable of a) safely mediating antitumor activity in the autologous and allogeneic HCT settings; b) persisting following adoptive transfer; c) expanding in response to CMV viral antigen in vivo (either vaccine-mediated or CMV-native); and d) sustaining the antitumor immune response long-term.These studies are significant in that they address poor post-HCT outcomes for patients with B cell NHL. Our approach is highly innovative in combining CAR T cells with CMV vaccine.
Specific Aim 1: Test bi-specific CMV-CD19CAR T cells manufactured to clinical scale as part of pre-IND studies.
Specific Aim 2: Pilot/feasibility studies of bi-specific autologous CMV-CD19CAR T cells +/- CMV vaccine following lymphodepletion or autologous HCT for intermediate grade CD19-positive NHL (Figure 2).
Specific Aim 3: Pilot/feasibility studies of donor-derived CMV/CD19 bi-specific T cells +/- CMV vaccine following allogeneic HCT for intermediate grade CD19-positive NHL.
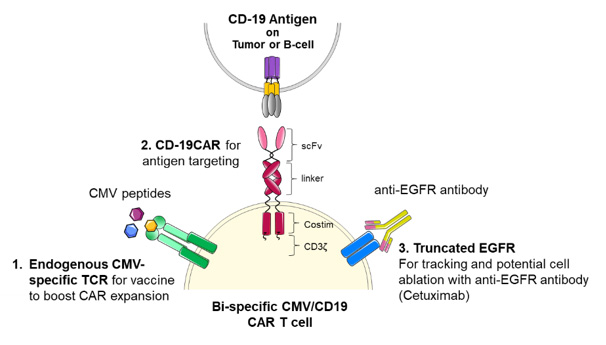
Figure 1. CMV-CD19 CAR T cell features
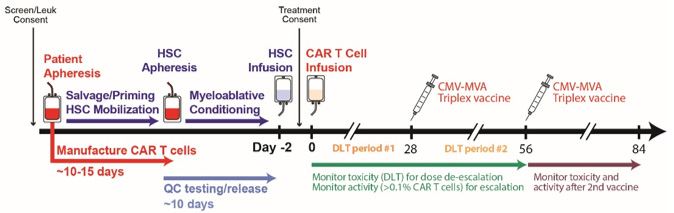
Figure 2. Treatment Schema for CMV-CD19CAR T cells with CMV vaccine following autologous transplant
Project 2: Therapy-related leukemia following autologous transplantation for lymphoma
Project Co-Leaders:
Ravi Bhatia, MD (Basic Science Co-Leader)
Smita Bhatia, MD (Clinical Science Co-Leader)
Stephen J. Forman, MD (Clinical Science Co-Leader)
Therapy-related myeloid neoplasm (t-MN) is a lethal complication of autologous hematopoietic cell transplantation (aHCT) for Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). t-MN is the leading cause of non-relapse mortality among aHCT recipients. It is generally believed that hematopoietic stem cells (HSCs) exposed to cytotoxic therapy suffer genomic damage and malignant transformation. However, high inter-individual variation in t-MN risk suggests a role for genetic susceptibility. We are conducting a genome-wide association study to identify germline variants associated with t-MN; top SNPs will contribute to creation of genetic profile. Somatic mutations in leukemia-associated genes DNMT3A, ASXL1, and TET2 seen in peripheral blood in ~10% of older healthy population, are associated with >10-fold increase in risk for subsequent leukemia; targeted next-generation sequencing will be used to identify driver mutations. We previously observed altered gene expression in PBSC samples from patients who subsequently developed t-MN and developed a 38-gene PBSC classifier, which will also contribute to the genomic profile. The elevated risk of t-MN after aHCT, coupled with the poor prognosis, present an unmet need for pre-aHCT identification of patients at increased risk for post-aHCT t-MN to guide use of alternative therapeutic options for HL/NHL management. We hypothesize that a combined clinical+genetic risk prediction model applied prior to aHCT will allow identification of HL/NHL patients at increased risk for post-aHCT t-MN. We will test this hypothesis through the following specific aims (depicted in Figure 3).
Aim 1: Create a clinical profile associated with t-MN in HL/NHL patients undergoing aHCT at COH (Discovery), using demographic/clinical characteristics, therapeutic exposures (pre-aHCT, aHCT-related)
Aim 2: Create a genetic profile associated with t-MN in HL/NHL patients undergoing aHCT at COH (Discovery), using the PBSC product for “38-gene” gene expression classifier, clonal somatic mutations in AML-associated genes and candidate germline SNPs from top GWAS SNPs/published SNPs
Aim 3: Use the Discovery Cohort (COH) to develop a prediction model that includes clinical profile, as well as genetic profile, to optimize identification of patients at high risk for developing t-MN.
Aim 4: Use an independent Validation Cohort (UMN+UNE) to test the validity of the optimized prediction model (Aim 1).
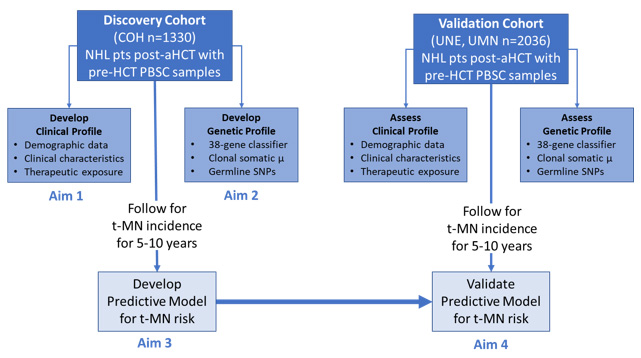
Figure 3. Experimental Design for Project 2
Project 3: Targeted immunotherapy for relapsed/refractory Hodgkin lymphoma
Project Co-Leaders:
Alex Herrera, MD (Clinical Science Co-Leader)
Anna Wu, PhD (Basic Science Co-Leader)
Joo Song, MD (Basic Science Co-Leader)
For patients with relapsed/refractory classical Hodgkin lymphoma (HL) after induction treatment, standard of care is 2nd-line combination chemotherapy followed by autologous hematopoietic cell transplantation (AHCT). Complete response (CR) at the time of AHCT is the strongest indicator of post-AHCT prognosis; thus, we have designed a clinical trial investigating enhanced second-line therapy to increase the proportion of patients in CR at the time of AHCT. The PD-1 inhibitor nivolumab (NIVO) has been approved for use in HL patients relapsing post-AHCT. In Specific Aim 1 we propose a multicenter phase II study evaluating NIVO followed by response-adapted addition of standard ifosfamide, carboplatin, etoposide (ICE) chemotherapy as a bridge to AHCT (Figure 4 Cohort A). This regimen, NICE, is designed to maximize CR rates pre-AHCT and spare many patients from salvage chemotherapy. We have also added a separate phase 2 cohort for patients with high-risk disease features (Figure 4 Cohort B) to estimate the activity of NIVO+ICE in this population. In Specific Aim 2 we focus on HL transplant therapy, augmenting the AHCT conditioning regimen with targeted radioimmunotherapy (RIT) to improve outcomes. This single-center phase II study combines standard BEAM (BCNU, etoposide, Ara-C, melphalan) chemotherapy with an Yttrium-90 (90Y)-labeled anti-CD25 antibody, basiliximab (Figure 5). Basiliximab directly targets radiation to CD25+ Reed Sternberg cells, as well as activated T cells and regulatory T cells (Tregs) throughout the HL tumor microenvironment. The crossfire effect of 90Y kills nearby cells including CD25-negative RS and immune cells in the tumor. The two studies are complementary, with NICE participants eligible for enrollment to the aTac-BEAM trial. The overarching goal of this project is to improve overall outcomes for patients with relapsed/refractory HL, via separate clinical trials to improve pre-AHCT CR rates and post-AHCT survival. Gene expression and tumor spatial analysis studies will help to elucidate the role of the TME in responses to anti-PD-1-based therapy.
Specific Aim 1: Response-adapted second-line therapy for Hodgkin lymphoma using anti-PD-1 antibody nivolumab ± ICE as a bridge to AHCT (NICE trial NCT03016871).
Specific Aim 2: 90Y-anti-CD25 radioimmunoconjugate combined with BEAM conditioning for AHCT in patients with relapsed/refractory Hodgkin lymphoma (aTac-BEAM trial NCT04871607).
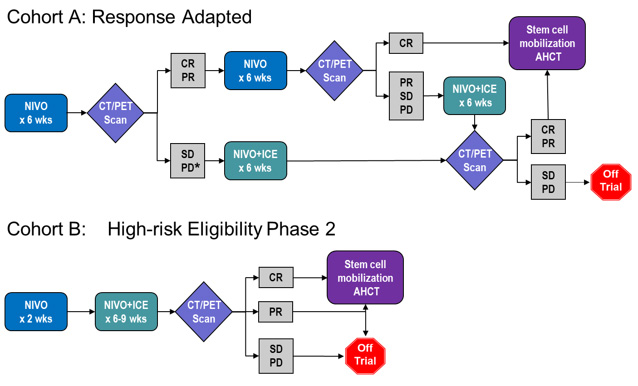
Figure 4. NICE Treatment Schema (Specific Aim 1)
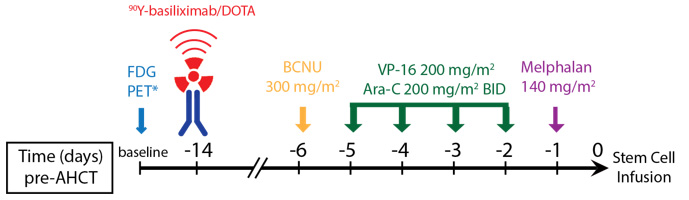
Figure 5. aTac-BEAM Treatment Schema (Specific Aim 2)
Project 4: Novel nucleotide-based approaches targeting the STAT3 pathway for the treatment of lymphoma
Project Co-Leaders:
Hua Yu, PhD (Basic Science Co-Leader)
Marcin Kortylewski, PhD (Basic Science Co-Leader)
Elizabeth Budde, MD, PhD (Clinical Science Co-Leader)
Growing evidence links the immune suppression and poor survival of B-cell lymphoma patients to persistent activation of signal transducer and activator of transcription 3 (STAT3). In ABC-DLBCL, genetic mutations augment Toll-like receptor 9 (TLR9)/MyD88 signaling, thereby leading to secretion of cytokines that induce STAT3 activation in lymphoma cells and in the tumor-associated immune cells, such as myeloid-derived suppressor cells (MDSCs). The autocrine and paracrine STAT3 activation in cancer cells and in the tumor-associated immune cells enhances lymphoma’s tumorigenic and tolerogenic potential. To overcome the challenge imposed by lack of pharmacological inhibitors of STAT3, we developed a strategy to deliver therapeutic siRNA specifically into myeloid and B cells, by physically linking siRNA to TLR9 ligands/agonists, CpG oligodeoxynucleotides (ODNs). We have demonstrated in multiple tumor models that local tumor treatment using CpG-STAT3siRNA silences STAT3, stimulating systemic antitumor immunity, in addition to inducing direct B-cell lymphoma tumor cell apoptosis and sensitivity to radiation therapy (Figure 6). We propose to move CpG-STAT3siRNA to phase 1 clinical trials in NHL. More recently as part of this SPORE project, we have developed a second generation STAT3 inhibitor: linking CpG to a high-affinity STAT3-DNA binding sequence called STAT3decoy ODN (dODN), which effectively competes with STAT3 for DNA binding. Compared to CpG-STAT3siRNA, CpG-STAT3dODN exhibits improved nuclease-resistance which allows for systemic administration and treatment of advanced, disseminated lymphomas. We anticipate that with the clinical testing of the first CpG-STAT3siRNA strategy together with RT in the local setting, and with the optimization of new CpG-STAT3dODN for systemic administration, the proposed studies will accelerate development of novel, effective and safe nucleotide-based immunotherapeutic strategies for targeting intracellular STAT3 in NHL and potentially other hematologic malignancies.
Specific Aim 1: Conduct first-in-human phase 1 trial of intratumoral injection of CpG-STAT3 siRNA in combination with local radiation in B-cell non-Hodgkin lymphoma (NCT04995536, Figure 7).
Specific Aim 2: Optimize the CpG-STAT3dODN strategy for targeting B cell lymphoma cells.
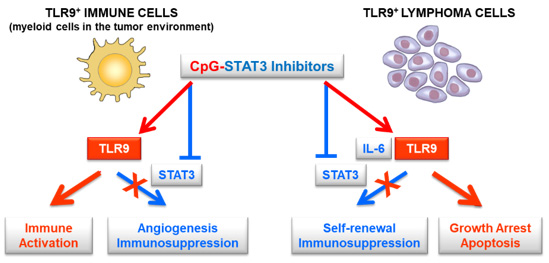
Figure 6. CpG-STAT3 inhibitors target both lymphoma cells and immune cells to simultaneously activate TLR9 and suppress STAT3
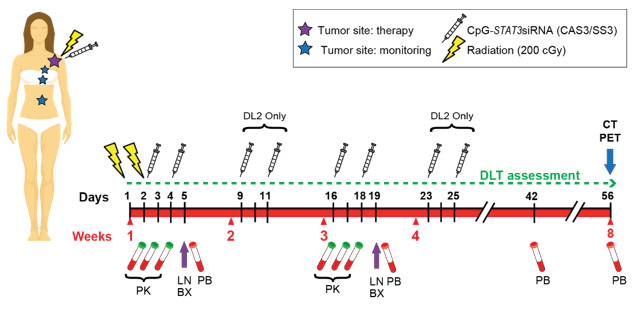
Figure 7. Treatment Schema for Phase 1 trial of CpG-STAT3 siRNA
Administrative Core
Core Directors:
Stephen Forman, MD
Larry Kwak, MD, PhD
Sandra Thomas, PhD
The Administrative Core directly supports management of SPORE projects, cores, and funds. The Core will coordinate SPORE-related meetings, preparing and submitting annual progress reports, and make decisions regarding selection and support of the best and most promising projects. Importantly, the Administrative Core will be responsible for the oversight of the Developmental Research and Career Development Programs in the Lymphoma SPORE. Finally, the Core will also promote patient interests and equitable access to treatment on SPORE studies by working with our patient advocate to ensure that SPORE clinical studies are patient centered.
Biostatistics and Bioinformatics Core
Core Directors:
Joycelynne Palmer, PhD
Core B, the Biostatistics and Bioinformatics Core of the City of Hope Lymphoma SPORE, provides information management and statistical expertise across all SPORE research activities, including study design, safety monitoring, data collection, data quality assurance, data analysis, and multicenter study site coordination. Core B will ensure that the proposed research hypotheses will be addressed with appropriate measures, tests, and interpretation, whether the data arise from genomic studies, basic science, translational, immunologic studies, or clinical trials. The centralized, comprehensive, and integrated framework of Core B assures each SPORE investigator access to statistical and informatics experts who have appropriate experience, interests and time to engage in the collaborative development of study designs, analysis plans, data analysis, interpretation, reporting, and abstract/manuscript preparation.
Core B will also provide infrastructure for the management and integration of both existing and newly collected data and specimens through consistent and compatible data handling. The Core plays an integral role in the scientific development, execution, and analysis of all projects in the SPORE. Core B investigators have extensive experience in quantitative methods for biomedical applications, including clinical, basic, and translational science studies, with particular expertise in hematologic malignancy research. Core B is committed to collaboration to help ensure the scientific integrity of the SPORE investigations, by participating in regular Project and Program meetings, and providing rigorous and innovative input on all statistical and information management matters arising within the projects.
The specific aims of Core B are as follows:
Specific Aim 1. To support high quality data management through state-of-the-art information technologies, ensuring standardization, quality assurance, security, training, monitoring, data integration, decision support, reporting and multicenter coordination.
Specific Aim 2. To provide biostatistical support to the Projects, Cores and Programs, by consulting on the design, data monitoring, analysis, statistical modeling, visualization, interpretation, reporting and publication of data generated by SPORE activities.
Tissue Bank Core
Core Directors:
John Chan, MD
Guido Marcucci, MD
The overarching goals of this core is to provide high quality, well annotated and strictly quality controlled biospecimens for the projects, to develop and implement assays needed for the analysis of the biospecimens and to collaborate with Core B to build the informatics infrastructures to facilitate all aspects of research linked to biospecimens.
Specific Aim 1 is to provide tissue banking and histology/pathology services. Specialized services will be performed according to the needs of the projects with the collection of leukapheresis specimens to generate CAR-T cells (Project 1); characterization of complex Hodgkin lymphoma and host cell populations through multispectral multiplexed immunofluorescence (mIF) phenotyping and gene expression profiling (GEP) (Project 3); cytogenetic and genomic characterization of lymphomas and the preparation of samples to generate primary patient-derived xenograft (PDX) non Hodgkin lymphoma models (Project 4).
Specific Aim 2: Because of the complexity of the datasets associated with the functions of Core C, we will collaborate with Core B (Biostatistics) to design, build, and integrate databases relevant to biospecimens to improve their functionality and connectivity to support SPORE projects and collaborations on other NCI-sponsored projects.
Manufacturing Core
Core Directors:
Larry Kwak, MD, PhD (Director)
David Horne, PhD (Co-Director)
The primary objective of Core D is to provide process development, regulatory support, and well-documented cGMP-compliant clinical-grade production of characterized CAR T cell products, monoclonal antibodies (mAb), lentivirus vectors, and complex small molecules, including nanomaterials, biopolymers (peptides, siRNA-aptamers, and DNA-peptide hybrids), and complex natural products. We apply our established project management, product development, regulatory, and manufacturing capability to the benefit of SPORE investigators by providing assistance in pre-clinical development strategy, regulatory affairs, and process devolvement. Quality Assurance, product characterization, and compliance with regulatory requirements for process and product gained from our extensive history of cGMP manufacturing for clinical trials will ensure suitability, control, and scalability of the proposed clinical projects.
Developmental Research Program
Program Directors:
Larry Kwak, MD, PhD
John Chan, MD
The purpose of the Developmental Research Program (DRP) is to identify and support first-rate, pioneering translational projects in lymphoma that, despite their potential, are not yet mature for full program status. All members of the research community at City of Hope and University of Alabama Birmingham (UAB) are encouraged to consider the opportunity. Up to two researchers per year receive awards, which are granted for up to two years. Promising investigations may replace SPORE research projects that have either acquired funding as independent R01/P01 projects, or those that have not satisfactorily progressed.
Career Enhancement Program
Program Directors:
Stephen J. Forman, MD
Michael Caligiuri, MD
The primary objective of the CEP is to attract and train quality investigators to the field of lymphoma translational research. This program will support, both financially and scientifically, junior or established investigators at City of Hope and University of Alabama Birmingham (UAB) who want to focus or refocus their research careers on lymphoma. Funds can also be used to recruit investigators who plan to pursue a career in lymphoma research to City of Hope and/or Beckman Research Institute, or University of Alabama. The CEP supports one to two individuals per year, with each awardee supported for a period of one to two years.







