Improving Outcome Disparities for Latino Children and Adolescents with Acute Lymphoblastic Leukemia
Baylor College of Medicine
Principal Investigator(s):

Karen Rabin, MD, PhD

Philip Lupo, PhD, MPH
- Principal Investigator(s) Contact Information
- Overview
- Project 1: Etiology and impact of ethnic disparities in therapy-associated hepatotoxicity among children and adolescents treated for acute lymphoblastic leukemia (ALL)
- Project 2: Ethnic disparities in methotrexate neurotoxicity among children and adolescents with acute lymphoblastic leukemia (ALL)
- Administrative Core
- Biospecimen Processing and Storage Core
- Biostatistics and Data Management Core
- Developmental Research Program
Principal Investigator(s) Contact Information
Karen Rabin, MD, PhD
Associate Professor of Pediatric Hematology-Oncology
Director, Leukemia Program
Texas Children's Cancer Center
Baylor College of Medicine
Texas Children's Cancer Center, 1102 Bates St, Suite 750.00
Houston, TX 77030
(832) 824-4213
Philip Lupo, PhD, MPH
Associate Professor of Pediatrics
Director, Epidemiology Program
Texas Children's Cancer and Hematology Centers
Baylor College of Medicine
Center for Epidemiology and Population Health
6620 Main Street
Suite 1100D
Houston, TX 77030
(713) 798-2960
Overview
The overall goals of this P20 program are to reduce outcome disparities among Latino children and adolescents with acute lymphoblastic leukemia (ALL) by identifying host biological factors that result in increased toxicities, and to lay the groundwork for establishment of the first Specialized Programs of Research Excellence (SPORE) devoted to pediatric leukemia.
Our P20 program includes two translational Research Projects.
Project 1. Etiology and impact of ethnic disparities in therapy-associated hepatotoxicity among children and adolescents treated for ALL
Project 2. Ethnic disparities in methotrexate neurotoxicity among children and adolescents with ALL
The Projects are supported by three shared resources: Core A. The Administrative Core; Core B. Biospecimen Processing and Storage; and Core C. Biostatistics and Data Management. The Program also supports a Developmental Research Program that will foster development of innovative pilot projects that aim to understand and/or reduce ethnicity-based disparities in ALL outcomes. We will leverage the Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, comprising 6 cancer centers in the southwestern U.S., which will provide clinical data from 3,000 and clinical data, bone marrow, blood and cerebrospinal fluid samples from nearly 2,000 children and adolescents with newly diagnosed ALL to investigate these two novel and integrated Projects.
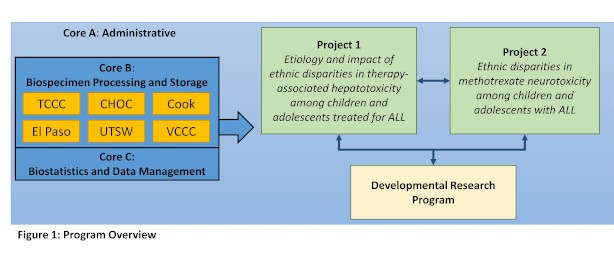
Project 1: Etiology and impact of ethnic disparities in therapy-associated hepatotoxicity among children and adolescents treated for acute lymphoblastic leukemia (ALL)
Project Co-Leaders:
Austin Brown, PhD, MPH (Basic)
Etan Orgel, MD
Van Huynh, MD (Clinical)
This proposal pursues the central hypothesis that risk of therapy-associated hepatotoxicity (TAH) is greater in Latino compared to non-Latino patients due to a combination of inherited and potentially modifiable risk factors. We seek to better understand ethnic disparities in the incidence and etiology of TAH by characterizing underlying genetic predisposition and prospectively monitoring differences in metabolomic profiles during ALL induction therapy using the multi-ethnic, multi-institutional Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, including a Retrospective (n=2,958), a Prospective (n=1,369), and a continuation P20 Cohort (n=600).
Specific Aim 1 will evaluate the role of obesity in ethnic differences in the incidence of TAH during induction therapy in patients
with pediatric ALL. We hypothesize that compared to non-Latino Whites, Latino children and adolescents have an increased risk for TAH during induction
therapy, which is further exacerbated in Latinos by their higher rates of obesity and non-alcoholic fatty liver disease (NAFLD).
Specific Aim 2 will identify pharmacogenomic and pharmacometabolomic profiles associated with ethnic differences in incidence of TAH
during ALL induction. We hypothesize that inherited genetic variants, which are enriched in individuals of Latino ancestry, alter the metabolomic response
to induction chemotherapy and the risk for developing TAH.
We anticipate that this study will define the elevated TAH risk found in Latino patients being treated for ALL and identify biological mechanisms responsible for this disparity. Ultimately, we expect these findings to inform risk-stratified approaches to minimize disparities in TAH in Latino children and adolescents treated for ALL.
Project 2: Ethnic disparities in methotrexate neurotoxicity among children and adolescents with acute lymphoblastic leukemia (ALL)
Project Co-Leaders:
Michael Scheurer, PhD, MPH (Basic)
Karen Rabin, MD, PhD (Clinical)
The antifolate agent methotrexate (MTX) is a critical component of ALL therapy. However, ~10% of pediatric ALL patients experience MTX-associated neurotoxicity, and management often involves treatment delays and/or modifications which may impact survival. Since preliminary data indicate that MTX neurotoxicity is more frequent in Latinos, the goal of this Project is to better understand factors contributing to ethnic disparities in MTX neurotoxicity. We hypothesize that germline genetics, tumor biology, and social determinants of health contribute to poorer outcomes.
Specific Aim 1 will compare the impact of acute MTX neurotoxicity on clinical treatment and outcomes between Latino and non-Latino Whites with ALL. We will utilize the ethnically diverse, well-annotated Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Retrospective Cohort to compare the incidence of initial and subsequent neurotoxicity between ethnically and ancestrally defined subgroups, and determine the contribution of neurotoxicity on treatment course and risk of relapse.
Specific Aim 2 will identify clinical, socioeconomic, pharmacogenomic, and metabolomic predictors of initial MTX neurotoxicity and neurotoxicity recurrence following MTX re-challenge in the REDIAL Prospective Cohort, while accounting for the impact of ethnicity. We will collect blood samples for germline pharmacogenomics and serial CSF samples during therapy for metabolomics analysis to identify groups at risk of poor outcomes based on clinical and molecular factors.
Specific Aim 3 will identify alterations in white matter integrity associated with initial MTX neurotoxicity and recurrence following MTX re-challenge, using standard and novel imaging techniques, in the context of ethnic variation. We will analyze existing clinical MRI scans from patients in the REDIAL Retrospective Cohort to identify predictors of initial MTX neurotoxicity and recurrence and to identify neuroimaging biomarkers that differ between Latino and non-Latino patients. We will also prospectively analyze a subset of patients using advanced MRI techniques and connectivity and tractography analysis to identify specific impacts on the neural network.
This project will provide insights into the factors responsible for increased neurotoxicity in Latino children with ALL and may identify pharmacogenomic and imaging features of at-risk individuals that allow prevention of initial or subsequent events, and improvement of ALL outcomes.
Administrative Core
Core Directors:
Karen Rabin, MD, PhD (Core Co-Director)
Philip Lupo, PhD, MPH (Core Co-Director)
Effective communication, administrative, and budgetary management are necessary to maximize the benefits of these efforts and ensure that the P20 Program achieves its larger long-term goals. The goals of the Administrative Core (Core A) are therefore to coordinate and organize the Projects and Cores, facilitate communication between Projects and Cores, and ensure continued adherence to the overall P20 Program goals.
By facilitating employment, training, regulatory compliance, internal and external communication, and financial management of the Program Project, Core A will maximize the productivity and efficiency of all participating Projects and Cores. Core A will also ensure regular internal communication among investigators and sites and organize external communication with leaders in the field, the scientific community, and patient advocacy organizations. In summary, the Administrative Core will ensure that all aspects of the proposed research program are conducted in accord with the highest standards of organization, documentation, communication, and fiduciary responsibility.
Biospecimen Processing and Storage Core
Core Directors:
Terzah Horton, MD, PhD (Core Co-Director)
Michael Scheurer, PhD, MPH (Core Co-Director)
The Biospecimen Processing and Storage Core will serve as the central location for processing of serial peripheral blood, bone marrow and cerebrospinal fluid (CSF) specimens from children and adolescents with acute lymphoblastic leukemia (ALL) across the participating clinical recruitment sites of the Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium, to generate the materials needed for the specific studies in Research Projects 1 and 2, as well as determination of genetic ancestry for use across all projects, and biobanking of material that will be available for pilot projects through the Developmental Research Program. This Core will support the collection, shipping, processing, and storage of patient samples from each study site, as well as provide up-to-date and accurate sample annotation. In summary, Core B will: 1) Receive and catalog blood, bone marrow and CSF provided by the Consortium sites; 2) Process all patient samples to isolate plasma, peripheral blood mononuclear cells, and bone marrow mononuclear cells, and separate blasts from non-blasts where appropriate; 3) Allocate and distribute samples to Projects; and 4) Maintain a biobank of samples for future research. The resulting biobank will become an extremely valuable resource for this and future translational studies examining ethnic disparities in childhood ALL.
Biostatistics and Data Management Core
Core Directors:
Hong Zhu, PhD (Core Co-Director)
Sandi Pruitt, PhD, MPH (Core Co-Director)
The Biostatistics and Data Management Core (Core C) functions as a centralized research design and data science coordination center for all P20 Projects, bringing together expertise and intellectual resources in biostatistics, bioinformatics, geospatial data analysis, and data management for all P20 investigators. Core C members will: 1) provide biostatistical and bioinformatics support for rigorous research design and state-of-the-science statistical analysis for all P20 Projects and the Developmental Research Program (DRP); 2) facilitate state-of-the-science geospatial data analysis for all P20 Projects and the DRP; and 3) develop and maintain an integrated database environment for efficient data management, integration, analysis and sharing for all P20 Projects, the DRP and investigator-initiated inquiries. Fully integrating Core C members into all P20 Projects and other Cores will build workflow efficiencies and support the translational goals of the P20, while enhancing quality and ensuring scientific integrity of participating Projects. Core C will provide sophisticated data management, design, and analysis services in order to facilitate important discoveries about acute lymphoblastic leukemia ethnic disparities and to guide future prevention and intervention strategies.
Developmental Research Program
Program Directors:
M. Monica Gramatges, MD, PhD (Co-Director)
Philip Lupo, PhD, MPH (Co-Director)
The objective of the DRP is to support the development and successful completion of innovative, high-risk/high-reward pilot projects that aim to understand or reduce outcome ethnicity-based disparities in childhood acute lymphoblastic leukemia (ALL). DRP-supported projects may be clinical or translational, but must address ethnic disparities in ALL treatment-related toxicities (acute or late onset). In addition to grant review and selection, the DRP Committee will be responsible for monitoring and oversight of funded projects via regular review of progress and final reports. The DRP's core mission is to provide grant awardees with 1) funding support, 2) access to core facilities, and 3) mentorship during the award period. A key role of the DRP is to facilitate the transition/development of Pilot Grant data to work that is independently funded, with potential inclusion in a future P50 SPORE application.







