Translational Research in Breast Cancer
Baylor College of Medicine
Principal Investigator:

Xiang Zhang, PhD
- Principal Investigator Contact Information
- Overview
- Project 1: Co-Targeting ER and Kinome Deregulation in Breast Cancers with Neurofibromin Deficiency
- Project 2: Targeting RTK Co-Dependencies in Triple-Negative
- Project 3: Targeting Endoplasmic Reticulum Stress Sensor IRE1 to Enhance Chemotherapy Sensitivity in MYC-driven breast cancer
- Administrative Core
- Pathology and Biobanking Core
- Informatics and Statistics Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigators Contact Information
Xiang Zhang, PhD
Interim Director, Lester and Sue Smith Breast Center
William T. Butler, M.D., Endowed Chair for Distinguished Faculty of Baylor College of Medicine
Professor, Molecular and Cellular Biology
Baylor College of Medicine
One Baylor Plaza
Houston, TX 77030
(713) 798-6239
Overview
Lack of progress in curing metastatic breast cancer is due to a number of fundamental treatment barriers. These include insufficient knowledge of therapeutic vulnerabilities, lack of reliable predictive biomarkers, inability to target common tumor suppressor gene loss, inability to target oncogenes that are not protein kinases or ligand- dependent transcription factors, resistance due to clonal evolution and tumor heterogeneity, and failure to mount an immune response against the tumor. This SPORE renewal focuses on these obstacles by articulating cross- cutting objectives and aligned approaches that increase the efficiency of core utilization and promote inter-project collaboration. The three full Projects include studies on how to target the derepressed kinases consequent upon loss of the PTPN12 or the NF1 tumor suppressors in breast cancer, and the development of a promising new therapeutic approach for MYC-positive breast cancer. During the execution of these Projects we will: a) deploy proteogenomic approaches for monitoring kinase targets and resistance pathways; b) establish high-quality biomarkers for clinical trial eligibility and stratification; c) investigate disease-monitoring approaches with circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA); d) incorporate immunological approaches into treatment regimens by increasing the quality and quantity of tumor infiltrating lymphocytes; e) embed our SPORE biomarker program into early phase clinical trials to inform the design of Phase 3 trials; and f) promote collaborative research between Academia, NCI-Supported Cooperative Groups, Industry, and Advocacy Groups.
These objectives are served by three reformatted Cores, with the addition of a dedicated and highly-qualified molecular research pathologist for the Pathology and Biobanking Core, the addition of deeper bioinformatics expertise to the Informatics and Biostatistics Core, and a new SPORE director plus additional advocates and advisory board members for the Administration Core. The Career Enhancement and Developmental Research Programs will be guided by highly experienced leadership and, as before, will cement the future of our SPORE. With this powerful enhanced program we will advocate nationally for progress in the treatment of advanced breast cancer, with the conviction that a cure is a near-term possibility.
Project 1: Co-Targeting ER and Kinome Deregulation in Breast Cancers with Neurofibromin Deficiency
Project Co-Leaders:
Eric Chang, PhD (Basic Co-Leader)
Bora Lim, PhD (Clinical Co-Leader)
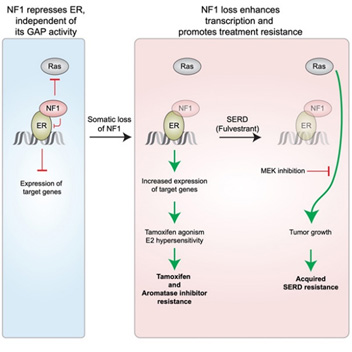
Over 250,000 cases of breast cancer are diagnosed annually in the US alone, and about 80% of these will be estrogen receptor-α positive (ER+). Despite great progress in treating ER+ breast cancer with endocrine therapy, treatment resistance remains a major problem, causing majority of deaths overall. Our clinical studies on endocrine therapy resistance have found that inactivation of the NF1 (for neurofibromatosis type 1) gene, which may occur in as many as 20% of primary ER+ breast cancer patients, correlates with poor outcome. NF1 encodes neurofibromin, best known as a Ras repressor (a GTPase Activating Protein, or GAP), but the scientific premise of this project stems from our discovery that NF1 has a GAP-independent activity by also acting as an ER transcriptional co-repressor. When the NF1-ER interaction is selectively inhibited (without affecting GAP activity), ER is de-repressed, estradiol (E2)-dependent gene expression is enhanced, and ER+ cells can grow in low E2 levels observed in aromatase inhibitor treated patients and are stimulated by tamoxifen, not inhibited. In contrast, NF1-depleted cells still respond to fulvestrant, a SERD (Selective ER Degrader), and although the reduced repression of Ras-Raf signaling in NF1-depleted cells promotes acquired resistance, which can be blocked by an FDA-approved MEK inhibitor (MEKi). This project will therefore investigate the hypothesis that NF1-deficient ER+ breast tumors should be treated by a SERD together with inhibitors blocking the Ras-Raf pathway. The key scientific premise is published in a Cancer Cell paper (Zheng et al 2020) and its graphic abstract is presented below:
Project 2: Targeting RTK Co-Dependencies in Triple-Negative Breast Cancer
Project Co-Leaders:
Thomas F. Westbrook, PhD (basic science leader)
C. Kent Osborne, MD (clinical science leader)
Xiang Zhang, PhD (basic science leader)
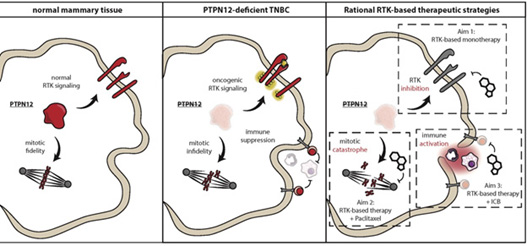
Despite extensive efforts to characterize the genomes and proteomes of triple-negative breast cancer (TNBC), no dominantly-acting mutated RTK has emerged as a therapeutic target in TNBC. Instead, TNBCs exhibit broad, rather than selective, hyper-activation of RTK signaling, with several proto-oncogenic RTKs hyper-activated in the same tumor. Recent discoveries have revealed that the coordinate activation of RTKs observed in TNBC is a result of inactivation of the protein tyrosine phosphatase PTPN12 (ex. Sun et al, Cell; Nair et al, Nature Medicine). In the current proposal, we aim to translate our new knowledge of the mechanisms by which RTKs are aberrantly activated and drive TNBC progression into a new therapeutic approach for TNBC patients.
Data from our team and confirmed by others indicates that PTPN12 functions as a tumor suppressor by restraining the activity of a select set of proto-oncogenic RTKs, including MET and PDGFRB, binding these receptors and suppressing downstream signaling. PTPN12 is compromised in 45-55% of TNBCs, and we have shown in in vitro and pre-clinical models of TNBC that inactivation of PTPN12 leads to hyper-activation of MET, PDGFRB, and a small subset of other RTKs. Importantly, this provokes the therapeutic hypothesis that PTPN12-deficient TNBCs can be treated by combined targeting of this select set of hyper-activated RTKs. To translate these pre-clinical findings into a new therapeutic approach, we will evaluate the efficacy of the well-tolerated MET/PDGFR inhibitor sitravatinib as monotherapy in metastatic TNBC patients, develop rational strategies to combine sitravitinib with standard of care therapies (based on newly discovered synthetic lethalities in TNBC), and explore new ways to capitalize on the anti-tumoral and pro-immune effects of sitravatinib in the context of immune checkpoint therapies.
Herein, our mechanistic, pre-clinical, and clinical studies will evaluate the proof-of-concept for the use of sitravatinib to treat PTPN12-deficient TNBC and lay the groundwork for the next generation of combination approaches for TNBC and other RTK-dependent cancers.
Project 3: Targeting Endoplasmic Reticulum Stress Sensor IRE1 to Enhance Chemotherapy Sensitivity in MYC-driven breast cancer
Project Co-Leaders:
Xi Chen, PhD (Basic Co-Leader)
Mothaffar Fahed Rimawi, MD (Clinical Co-Leader)
Xiang Zhang, PhD (Basic Co-Leader)
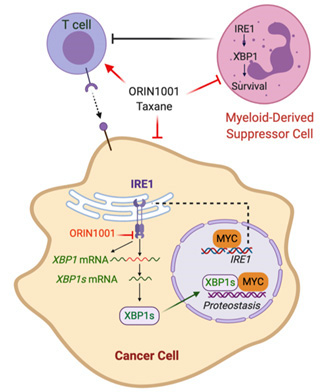
Limited sensitivity to chemotherapy and an immunosuppressive tumor microenvironment drives breast cancer mortality in HER2 negative breast cancer. We have identified the IRE1/XBP1 branch of the Unfolded Protein Response (UPR) or endoplasmic reticulum (EnR) stress response as a previously unexplored therapeutic vulnerability in MYC-driven breast cancer that enhances chemotherapy sensitivity and reverses the immunosuppressive tumor microenvironment. IRE1 is amplified in ~10% of human breast cancer, and frequently co-amplified with the MYC oncogene. Activation of MYC is synthetic lethal with IRE1/XBP1 pathway inhibition. We found that inhibition of the IRE1/XBP1 pathway with a highly selective IRE1 RNase inhibitor ORIN1001 suppresses MYC-high-expressing (MYChigh), but not MYC-low-expressing (MYClow), tumor growth in patient-derived xenograft (PDX) models. ORIN1001 substantially enhances the docetaxel efficacy, resulting in rapid tumor regression of the MYChigh PDX tumors. Furthermore, the ORIN1001 plus docetaxel therapy triggers massive cytotoxic T-cell infiltration, depletion of immunosuppressive myeloid-derived suppressor cells (MDSCs) and substantial upregulation of PD-L1 in the tumor microenvironment. ORIN1001 has excellent safety profile and is well-tolerated. These preclinical data prompt us to hypothesize that inhibition of the IRE1/XBP1 pathway with the IRE1 inhibitor ORIN1001 compromises MYChigh breast cancers by inhibiting obligatory UPR stress adaptation required for cellular viability. This therapeutic effect of ORIN1001 is associated with a marked increase in taxane sensitivity. In addition, MDSCs are also selectively depleted by ORIN1001 in the presence of a taxane, thus reversing immunosuppression and potentially sensitizing MYChigh breast cancers to immune checkpoint intervention. A pre-clinical phase study in breast cancer models will be conducted in parallel with a Phase 1 clinical trial. The overarching objective of this proposal is to design and open a Phase 2 clinical trial in MYC-positive metastatic breast cancer with eligibility and endpoints to be defined by the execution of the research aims.
Administrative Core
Core Directors:
Xiang Zhang, PhD (Core Director)
Eric Chang, PhD (Co-Core Director)
Bora Lim, MD (Clinical Science Leader)
The Administrative Core is charged with facilitating communication and sustaining integration among the SPORE members and projects, as well as providing efficient support services for all the SPORE components, in order to maximize synergy in achieving our translational research goals.
Pathology and Biobanking Core
Core Directors:
George Miles, MD, PhD
Carolina Gutierrez, MD
The Pathology and Biobanking Core will provide the following services to projects and integration with other cores in this proposal: 1) A centralized resource to acquire, store, and utilize human biospecimens for cancer research; 2) To coordinate and manage the pathology activities of this SPORE project, and ensure that Project Investigators have ready access to specialized pathology expertise and interpretation; and 3) To design and validate biomarker-based tests (genomic and/or proteomic) that support all project stages, including clinically-validated and reported assays for SPORE-based clinical trials.
This Core seeks to improve the lives of breast cancer patients by facilitating novel translational biomarker discovery into the development and validation of clinical assays. This Core serves as a centralized repository for biospecimen acquisition and use, management of the histopathology activities of each SPORE project, and the development and validation of biomarker-based molecular testing that supports all stages of SPORE project development.
Informatics and Statistics Core
Core Directors:
Susan Hilsenbeck, PhD
Bing Zhang, PhD
Meenakshi Anurag, PhD
The Informatics and Statistics Core (ISC) will provide three broad types of services: (1) Comprehensive biostatistical consultation, experimental design, data analysis and reporting; (2) Integrative proteome-genomic bioinformatic consultation, experimental design, data analysis and reporting; (3) Development, customization, integration, and maintenance of databases and data management systems to support data management needs of SPORE projects and cores. As a by-product, when necessary, the Core can also develop new methods or adapt existing methods from other arenas to meet the unique needs of the SPORE.
Developmental Research Program
Program Directors:
Jeff Rosen, PhD
Alastair Thompson, MD, FRC, SEd
In order to enable SPORE investigators to rapidly develop new research opportunities which could translate into early benefits for breast cancer patients, and to allow for exploration of new techniques which may require substantial efforts but which are nevertheless not ready for full scale multi-year research funding, we have devoted considerable effort and resources to this SPORE Developmental Research Program.
Career Enhancement Program
Program Directors:
Suzanne Fuqua, PhD
C. Kent Osborne, MD
Awardees will participate in translational breast cancer research projects. There are one to two awardees at any one time, who may be either MD’s or PhD’s. Candidates are selected based on their previous accomplishments and their potential and desire to pursue a career in academic breast cancer research. While our primary focus is to support promising young investigators at the junior faculty level, it is possible that more established investigators may also be appropriate for support.
Institutional SPORE Website
https://www.bcm.edu/academic-centers/lester-and-sue-smith-breast-center/research/spore







