Yale Head and Neck Cancer SPORE: Overcoming Treatment Resistance in Head and Neck Cancer
Yale University
Principal Investigator:

Barbara Burtness, MD
- Principal Investigator Contact Information
- Overview
- Project 1: Improved Targeting of EGFR Family Members in Squamous Cell Carcinomas of the Head and Neck
- Project 2: Synthetic Lethal Therapy for HPV-Negative Head and Neck Cancer
- Project 3: Demethylation of HPV-associated head and neck cancer to trigger APOBEC synthetic lethality and enhance immune response
- Administrative Core
- Bioinformatics and Biostatistics Core
- Biospecimen Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigator Contact Information
Barbara Burtness, MD
Professor of Medicine
Interim Assoc Cancer Center Director
Diversity, Equity, and Inclusion Disease Aligned Research Team Leader, Head and Neck Cancers Program
Yale Cancer Center
25 York Street, PO Box 208028
New Haven, CT 06520-8028
(203) 737-7636
Overview
The Yale Head and Neck SPORE (YHN-SPORE) represents highly translational researchers with deep disease-based expertise who leverage the extraordinary scientific strength at Yale Cancer Center, in collaboration with investigators at Fox Chase Cancer Center and the University of North Carolina Lineberger Cancer Center, to improve treatment for patients with head and neck cancer. YHN-SPORE investigators have significantly impacted the field of HNSCC through training, and translational and clinical research. Basic scientists bring rigorous methodology to bear.
The YHN-SPORE seeks to address critical barriers to cure of HNSCC due to resistance to immune, DNA damaging and targeted therapy through these specific aims:
Aim 1: To overcome resistance to EGFR inhibition in HNSCC by targeting active conformations of ErbB family members;
Aim 2: To advance rational synthetic lethal combination therapy to the clinic in HPV-negative HNSCC;
Aim 3: To advance combination demethylating therapy with immune checkpoint inhibition to the clinic for HPV-mediated HNSCC, with mechanistic studies and characterization of immune response;
Aim 4: To bolster the foundation for HNSCC research through our Administrative, Biospecimen and Biostatistics/Bioinformatics cores, to engage institutional resources and the wider SPORE community; and
Aim 5: To advance new research and to foster the next generation of HNSCC translational researchers through a Developmental Research Program, a Career Enhancement Program, and interaction and collaboration with the wider SPORE and HNSCC research communities.
The overarching theme of the 3 coordinated projects is overcoming treatment resistance, spanning mechanistic insights into resistance to current treatment modalities and immunotherapy; translational validation; human endpoints to underpin future trials of novel strategies to circumvent resistance; mechanistic confirmation in correlative studies; and clinical trials in HPV-negative and HPV-driven HNSCC. Anticipated translational outcomes of the YHN-SPORE are:
(1) conformationally sensitive inhibitors to overcome resistance to EGFR inhibition in HNSCC;
(2) clinical safety and pharmacodynamic data combined aurora A kinase/WEE1 inhibition in HPV-negative HNSCC;
(3) proof-of-concept and immuno-profiling data to support development of combined demethylation and immunotherapy in HPV-mediated HNSCC;
(4) novel models and genomically-characterized tumors to enable HNSCC translational research; and
(5) a diverse group of young investigators who will emerge as the generation who cure HNSCC.
Project 1: Improved Targeting of EGFR Family Members in Squamous Cell Carcinomas of the Head and Neck
Project Co-Leaders
Kathryn Ferguson, PhD (Basic Co-leader)
Joseph Contessa, MD, PhD (Clinical Co-leader)
A significant challenge for improving epidermal growth factor receptor (EGFR) targeted therapies is to identify and clinically validate actionable mechanisms of therapeutic resistance. In this proposal, we have designed a strategy that iterates between basic science investigations, preclinical testing, and clinical specimen testing to elucidate the mechanism of cetuximab resistance in head and neck squamous cell carcinoma (HNSCC). Using an in vitro approach for analyzing cetuximab resistance, we identified upregulation of a targetable autocrine ligand, NRG-1, as a target mechanism of resistance. We have modeled this resistance both in cell lines and in vivo, using mouse xenograft studies, and have shown that it can be reversed therapeutically by using an ErbB3-targeted antibody therapeutic (CDX-3379) — which can restore responses to cetuximab and radiation therapy. We have also observed NRG-1-induced resistance to small molecule EGFR kinase inhibitors in cancer cells, and have studied the mechanistic origin of this resistance at a structural level. We propose to exploit this new knowledge to advance small molecular approaches for targeting EGFR family members in HNSCC. In parallel with these studies, we will study clinical specimens from an ongoing phase II HNSCC trial of afatinib plus cetuximab, plus two ECOG trials of cetuximab, to investigate resistance mechanisms in the clinic. We will also develop patient-derived xenografts (PDX) models from the ongoing clinical trial to test hypotheses for resistance mechanisms and to assess effectiveness of new strategies devised to overcome it. The key premise of the proposal is that understanding mechanisms of resistance to cetuximab will open up new therapeutic opportunities — allowing us to develop approaches that can still inhibit EGFR when cetuximab fails, and to develop approaches to target other molecules that activate EGFR in a cetuximab-insensitive way (such as ErbB3).
Project 2: Synthetic Lethal Therapy for HPV-Negative Head and Neck Cancer
Project Co-Leaders:
Erica Golemis, PhD (Basic Co-leader)
Barbara Burtness, MD (Clinical Co-leader)
HPV- HNSCC typically lose G1/S cell cycle checkpoints, with most tumors having mutations in TP53, and many also mutating other tumor suppressors such as CDKN2A. Such tumors are highly dependent on the G2-M checkpoint, providing a tumor-selective vulnerability to synthetic lethal strategies controlling progress through G2/M. In extensive preliminary data, we showed that adavosertib, an inhibitor of the G2/M checkpoint kinase WEE1, potently sensitizes TP53mut HNSCC cell lines to inhibition of Aurora A kinase (AURKA). AURKA inhibition upregulates WEE1, inducing an inhibitory Y15-phosphorylation of CDK1, blocking M-phase entry. WEE1 inhibition abrogates this arrest, accelerating mitotic entry for cells bearing highly disruptive spindle abnormalities and other defects arising from AURKA inhibition, resulting in mitotic catastrophe and apoptosis. We found combined AURKA/WEE1 inhibition is potent in HNSCC xenografts, while well-tolerated in normal tissue. We have extended this concept, identifying additional promising drug combinations between WEE1 and other G2/M regulatory kinases (PLK1 and CHK1). Notably, the limited number of cells surviving synthetic lethal treatment are characterized by aneuploidy and other defects suggesting they may have increased tumor mutation burden (TMB), express neoantigens, and upregulated inflammatory signaling associated with sensitivity to immune checkpoint inhibition.
This project will take this observation directly to the clinic. We will conduct a pre-operative window phase I and expansion clinical trial of the late generation, high potency selective AURKA inhibitor VIC1911 with adavosertib combination, establishing pharmacodynamic proof of concept, identifying biomarkers for patient selection in future studies, and placing synergistic combinations in context of genomic alteration and treatment resistance.
Aim 1: We will evaluate mechanisms of combination lethality, and use single cell sequencing and Luminex profiling to query TMB, predict neoantigens, and measure inflammatory signaling.
Aim 2: Will query the effect of classes of common HNSCC TP53 and CDKN2A mutations, and cisplatin resistance, on response to a WEE1-AURKA inhibitor combination, using defined cell line models and patient derived xenografts (PDXs).
Aim 3: We will perform a pre-operative window trial to establish recommended phase II doses, determine activity and establish pharmacodynamic proof of concept, and to evaluate putative predictive biomarkers for response to combination WEE1/AURKA inhibition in HNSCC.
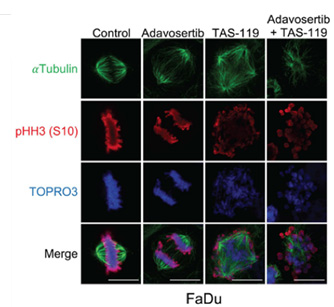
Photo Caption: Mitotic phenotypes with single agent or combination therapy. FaDu cells treated with adavosertib (inhibits WEE1), TAS119 (inhibits AURKA with high specificity), or combination. Adavosertib accelerates mitotic entry with accumulation in late mitosis. TAS119 leads to multipolar spindle formation and cell cycle arrest. Combination therapy precipitates mitotic catastrophe as cells with disrupted spindles enter mitosis which they are unable to complete.
Project 3: Demethylation of HPV-associated head and neck cancer to trigger APOBEC synthetic lethality and enhance immune response
Project Co-Leaders:
Karen Anderson, PhD (Basic Co-leader)
Wendell Yarbrough, MD (Clinical Co-leader)
Human papillomavirus (HPV)-associated neck squamous cell carcinoma (HNSCC) represents an increasing proportion of HNSCC. Based on observed hypermethylation of HPV+ HNSCC from TCGA, and understanding that HPV uses hypermethylation to impede the innate immune response, effects of the demethylating agent, 5-azacytidine (5-azaC), were tested on HPV+ HNSCC. We found that HPV+ HNSCC cells in culture and xenografts are sensitive to 5-azaC, and that 5-azaC caused double strand breaks (DSB) that were not observed after 5-azaC therapy in HPV-negative HNSCC, even with much higher doses. We found that following 5-azaC therapy, APOlipoprotein B mRNA-Editing enzyme Catalytic polypeptide 3B (APOBEC3B) was associated with chromatin in HPV+ HNSCC, but not HPV-negative cells. CRISPR knockdown of A3B prevented DSB and protected cells from 5-azaC-induced death. Despite being required for DSBs and cellular toxicity caused by 5-azaC, A3B was also required for clonogenic survival of untreated HPV+ HNSCC. These data showing that A3B is required for survival of HPV+ HNSCC cells, but that following demethylation A3B mediates toxicity and DSB. In addition, 5-azaC therapy increased type I interferon signaling as measured by increased expression of interferon-stimulated genes. These exciting pre-clinical data led to a window trial of 5 days of 5-azaC. Analysis of tumor specimens confirmed in vitro data showing that 5-azaC resulted in cellular toxicity. Immunofluorescent staining of an HPV+ patient tumors pre- and post-5-azaC showed a marked increase in tumor-associated lymphocytes, possibly driven through activation of type I interferon combined with increased expression of neoantigens. In this YHN-SPORE project, we hypothesize 5-azaC therapy will enhance response to nivolumab through its ability to cause cell death, increase neoantigen expression, increase A3B-driven mutational load, and enhance T cell infiltration through increased type I interferon signaling.
Aim 1: Tumor specimens from the SPORE window trial will be analyzed to determine effects of 5-azaC, nivolumab, or the combination on cell death, cell proliferation, immune infiltration and immune activation.
Aim 2: We will explore the role of A3B in cellular toxicity exposed by 5-azaC therapy.
Aim 3: We will determine effects of 5-azaC on activators of immune recognition and response in the presence or absence of nivolumab.
Administrative Core
Core Directors:
Barbara Burtness, MD
Anna Arnal Estapé, PhD
The Administrative Core (Core A) will be directed by Dr. Barbara Burtness, Principal Investigator of the Yale Head and Neck SPORE in Lung Cancer (YHN-SPORE), and co-directed by Dr. Anna Arnal Estapé. Drs. Erica Golemis and Wendell Yarbrough will serve as Site Leaders for Fox Chase Cancer Center and University of North Carolina, respectively. Careful oversight by the Administrative Core will be critical to ensure the success of the YHN-SPORE. The Administrative Core will serve as the central coordination point for all YHN-SPORE investigators, with responsibility for strategic and scientific direction, and monitoring progress of all projects and cores towards the translational goals of the SPORE. The Core Director and Co-Directors will have responsibility for leading the SPORE, setting translational research priorities, identifying new translational research opportunities, monitoring the progress of all projects, cores and developmental projects and determining changes in direction of projects, cores and translational research as needed. Interactions among YHN-SPORE investigators will be facilitated by Core A to accelerate the translation of laboratory findings to the bedside through rational and rigorous translational and clinical studies. If required, the Core A Director will manage conflicts among SPORE investigators. As a team, the Core personnel will provide financial oversight, maintain communication among project and core leaders and coordinate bi-weekly meetings, quarterly meetings of the Executive Committee, annual meetings of the Internal/External Advisory boards and an annual YHN-SPORE retreat. In addition to these functions, the Core will be the primary interface with the NCI, Fox Chase Cancer Center, University of North Carolina, head and neck or other disease- or pathway-focused SPOREs, Yale Cancer Center and Yale School of Medicine, and will coordinate outreach efforts, including publications (internal and external), website development, seminars, and patient/research advocacy activities. Through these administrative activities, Core A will be essential to the organization of the YHN-SPORE program, to advancing science to overcome treatment resistance in head and neck cancer, developing models and approaches to advance the field, and mentoring a new generation of head and neck cancer translational researchers.
Bioinformatics and Biostatistics Core
Core Directors:
Jeffrey Townsend, PhD
Eric Ross, PhD
The goal of the Biostatistics and Bioinformatics Core (Core C) is to address the statistical design and analysis needs of the Yale SPORE in Head and Neck Cancer (YHN-SPORE) Projects, the Cores, the Developmental Research Program (DRP), and the Career Enhancement Program (CEP). The specific aims of the Biostatistics and Bioinformatics Core are:
Aim 1: Provide collaboration and consulting in the design, execution, monitoring and analysis of basic, translational, population, and clinical studies for YHN-SPORE.
Aim 2: Oversee and coordinate the collection, management, and analysis of data. Ensure that data collected on all YHN-SPORE studies are of high quality, are evaluated with bioinformatic and statistical rigor, are accessible within SPORE-affiliated collaborative groups, ultimately enabling the widest accessibility that is appropriate given publication and privacy concerns.
For Aim 1, the Core will work closely with the investigators to analyze clinical trial and pre-clinical data, next generation sequencing data, and bioinformatics data mining. Services provided by the Core will range from planning and design activities to consulting on specific analytic questions. The Core will address the analytic and informatics questions arising from the YHN-SPORE projects. The Core will rigorously design and monitor clinical trials for safety, efficacy and futility. The Core will schedule regular meetings with the YHN-SPORE investigators, and maintain an open-door policy for any biostatistical and bioinformatics questions. The Core will conduct interim and final analyses, develop new statistical and bioinformatics methodology as needed, provide timely suggestions to YHN-SPORE investigators, and thus play an important role in the entire study.
For Aim 2, The Core will work with the Yale Cancer Center (YCC), Yale Center for Analytical Sciences (YCAS), Fox Chase Cancer Center (FCCC) and its quantitative cores to facilitate communication among YHN-SPORE researchers, creating and implementing web-accessible databases for collecting, storing and accessing the various types of data (e.g. laboratory, clinical, population, and genomic data) that will be generated for the YHN-SPORE projects.
Biospecimen Core
Core Directors:
David Rimm, MD, PhD
Denise Connolly, PhD
The Biospecimen Core B is designed to support all aspects of the Yale Head and Neck SPORE that need to use or analyze biospecimens. We have 4 specific aims that are designed to span the needs of the projects and include potential future work, including the DRPs and CEPs. Our aims include:
Aim 1: To collect, store and distribute human biospecimens with complete clinical, pathological, and demographic annotation.
Aim 2: To conduct or assist in the collection, maintenance and distribution of head and neck squamous cell carcinomas (HNSCC) cell lines.
Aim 3: To conduct or assist in preparation for genomic analyses of biospecimens.
Aim 4: To conduct or assist in the in situ molecular pathology analyses of biospecimens. Overall, we look forward to serving as a key tissue provider and analysis facility to allow the SPORE projects to seamlessly generate data from the Yale and Fox Chase Cancer Center tissue resources.
Developmental Research Program
Program Directors:
Roy Decker, MD, PhD
Barbara Burtness, MD
Karen Anderson, PhD
The primary goal of the Developmental Research Program (DRP) of the Yale SPORE in Head & Neck Cancer (YHN-SPORE) is to identify and fund innovative pilot projects that possess translational potential to make an impact in the field of head & neck cancer in the areas of risk assessment, early detection, biomarkers for prognosis and therapy prediction, mechanisms of carcinogenesis/tumorigenesis, novel therapeutic targets, development of novel therapeutics, and novel treatment approaches. Investigators of funded developmental research projects are strongly encouraged to collaborate with other investigators within and outside of the YHN-SPORE institutions, including other SPORE communities. The purpose is to provide each funded DRP project with the potential to evolve into independent full SPORE projects or equivalent-scale study proposals. Ultimately, along with the main YHN-SPORE projects, the outcome from this program will contribute to the reduction of head & neck cancer morbidity and mortality in the US and worldwide.
Career Enhancement Program
Program Director:
Kathryn Ferguson, PhD
A critical element to ensure new advances that translate research from bench to bedside in head and neck cancer is to attract, foster and support new investigators in this area. The Career Enhancement Program (CEP) of the Yale Head and Neck SPORE has this as its core goal. The communities of Yale, University of North Carolina and Fox Chase Cancer Center provide an ideal environment to promote this program. Each institution is strongly committed to promoting basic, translational and clinical cancer research. There is an exceptional pool of junior faculty, fellows and established investigators with outstanding potential to advance translational head and neck cancer research, plus a dedicated cohort of established head and neck investigators to serve as mentors and collaborators. To ensure that there will be a pipeline of new, improved and much needed therapies to offer to head and neck cancer patients, it is essential to increase the workforce of translational researchers who are driven to gain greater understanding of this disease and motivated to develop new treatments to reduce head and neck cancer morbidity and mortality. To achieve this the specific aims of the CEP are:
Aim 1: to identify, support and mentor promising early career basics and translational investigators to work in head and neck cancer,
Aim 2: To enhance the careers of established investigators who have newly-defined interest in pursuing translational research in head and neck cancer, and
Aim 3: to contribute to the development of a diverse head and neck cancer translational work force. This is of particular importance for a cancer which disproportionately burdens communities of color, such as those in the communities surrounding our cancer centers in New Haven, Chapel Hill and Philadelphia. The awardees will be drawn for the Yale, UNC and FCCC communities, selected through a careful and well-defined process, and supported by the expertise and resources of the YHN-SPORE community. These CEP awardees will go on to take their place in the next generation of physician scientists and translational researchers dedicated to addressing the most urgent issues in head and neck cancer.







