The University of Texas MD Anderson Cancer Center SPORE in Ovarian Cancer
The University of Texas MD Anderson Cancer Center
Principal Investigator(s):

Anil Sood, MD

Robert Bast, MD
- Principal Investigator(s) Contact Information
- Overview
- Project 1: The SIK2 Inhibitor GRN-300 Enhances PARP Inhibitor Sensitivity and Cytotoxic T-Cell Function in Ovarian Cancer
- Project 2: Next-Generation Engineered NK Cell Immunotherapy for Ovarian Cancer
- Project 3: Targeting EGFL6 in Ovarian Cancer
- Project 4: Targeting p300 to Overcome PARP inhibitor resistance induced by acidic tumor microenvironment
- Administrative Core
- Bioinformatics and Biostatistics Core
- Pathology Core
- Developmental Research Program
- Career Enhancement Program
Principal Investigator(s) Contact Information
Anil Sood, MD
Professor, Department of Gynecologic Oncology and Reproductive Medicine
Director, Ovarian Cancer Research, Department of Gynecologic Oncology
Co-Director, Center for RNA Interference and Non-Coding RNA
Director, Blanton-Davis Ovarian Cancer Research Program
Co-Director, Ovarian Cancer Moonshot Program
University of Texas MD Anderson Cancer Center
1515 Holcombe Blvd.
Unit 136
Houston, Texas, 77030
(713) 745-5266
Robert Bast, MD
Vice President for Translational Research
Director, Department of Translational Molecular Pathology
Internist and Professor of Medicine, Department of Experimental Therapeutics
Harry Carothers Wiess Distinguished University Chair for Cancer Research
University of Texas MD Anderson Cancer Center
1515 Holcombe Blvd.
Unit 1950
Houston, Texas, 77030
(713)-792-7743
Overview
The overall goal of the MD Anderson Cancer Center SPORE in Ovarian Cancer is to test and translate novel therapeutic strategies, including those to overcome adaptive resistance to conventional cytotoxic chemotherapy, poly (ADP-ribose) polymerase inhibitors (PARPi), anti-angiogenic agents (bevacizumab) and immune checkpoint blockade. We have successfully implemented measures to increase recruitment of women and underrepresented minorities to our Developmental Research Program (DRP) and Career Enhancement Program (CEP). Over the last 22 years, our SPORE investigators have been highly productive with major discoveries. These include: 1) conducted the SPORE and EDRN-supported Normal Risk Ovarian Screening Study (NROSS) where 71% of cases were detected in stage I or II; 2) identified biomarkers that detect 18% of CA125 negative cases; 3) developed a 4-biomarker algorithm that detects advanced stage disease earlier than the CA125-based NROSS algorithm; 4) found anti-TP53 autoantibodies elevated 8 months before CA125 and 22 months before diagnosis; 5) observed a 54% objective response rate to anti-angiogenic therapy with aflibercept and docetaxel; 6) completed a trial targeting Dll4; 7) demonstrated that CSF1R inhibitors can deplete macrophages and reduce resistance to anti-VEGF therapy; 8) demonstrated significant activity of selumetinib in low-grade ovarian cancers and completed an international phase III trial of another potent MEK inhibitor trametinib; and 9) developed a robust biomarker panel that predicts response to PARPi and completion of multiple trials combining PARPi with rationally selected drugs in high-grade ovarian cancer.
The SPORE consists of 4 projects:
Project 1 and Project 4 investigators tackle therapeutic resistance to PARP inhibitors and immune checkpoint blockers from multiple directions to speed progress and improve outcomes for women with ovarian cancer. Both projects have the potential to enhance T-cell infiltration in tumors and impart immunologic memory, which is particularly important given the likelihood of this cancer to recur.
Project 2 will develop a novel TROP2-targeted CAR-NK therapy.
Project 3 will develop therapy aimed at the tumor microenvironment using a novel EGFL6 targeted monoclonal antibody. Overall, our translational studies conducted in an optimal environment with a multi- institutional team are directed toward improving clinical outcomes of women with ovarian cancer.
Project 1: The SIK2 Inhibitor GRN-300 Enhances PARP Inhibitor Sensitivity and Cytotoxic T-Cell Function in Ovarian Cancer
Project Co-Leaders:
Robert Bast, Jr., MD (Basic)
Zhen Lu, MD (Basic)
Amir Jazaeri, MD (Clinical)
Poor outcomes for patients with ovarian cancer relate to delayed diagnosis and development of resistance to conventional therapy with carboplatin and paclitaxel. In the last SPORE cycle, we evaluated a novel inhibitor of salt-induced kinase 2 (SIK2) GRN-300 that enhances sensitivity to both carboplatin and paclitaxel, initiating a first-in-human phase IA/B trial to define the maximum tolerated dose of GRN-300 alone and in combination with weekly paclitaxel. Preclinical studies demonstrated that GRN-300 enhanced olaparib sensitivity in homologous recombination (HR)-proficient and deficient ovarian cancer cell lines and xenografts. We found that GRN-300 enhances olaparib sensitivity by 1) abolishing the class IIa histone deacetylase 4/5/7-associated transcriptional activity of myocyte enhancer factor 2D (MEF2D), 2) decreasing MEF2D binding to regulatory regions with high chromatin accessibility in DNA repair genes, and 3) repressing critical gene expression in the DNA repair pathway (Figure 1). Whereas poly(ADP-ribose) polymerase inhibitors (PARPi) have played a large part in maintaining progression-free survival in patients with HR-deficient ovarian cancers, the majority of patients will have resistance to PARPi and experience relapse. Moreover, combining conventional or other targeted agents with PARPi has been limited by additive myelosuppression. To date, GRN-300 has had no significant marrow toxicity in our phase I clinical trial. GRN-300 enhances olaparib activity in both olaparib-sensitive and acquired olaparib-resistant ovarian cancer cells. Cancer immunotherapy with immune checkpoint blockade (ICB) has had only limited impact on ovarian cancers. We have found that GRN-300 increases phosphorylation of TBK1 and nuclear localization of IRF3 in murine ovarian cancer cells. Both TBK1 and IRF3 are downstream targets of the cGAS/STING pathway (Figure 2). GRN-300 or GRN-300 combined with olaparib increases the expression of programmed death-ligand 1 (PD-L1) in human and murine ovarian cancer cells. GRN-300 combined with anti-PD-L1 enhances CD8+ T-cell infiltration and antitumor activity in a syngeneic ovarian cancer model. We will pursue three aims: 1) to perform a phase IB trial of GRN-300 in combination with a PARPi, 2) To determine the underlying mechanisms of olaparib resistance that can be overcome with the SIK2 inhibitor GRN-300 in combination with olaparib in ovarian cancer cell lines, xenografts and PDXs, and 3) To identify the mechanism(s) by which the SIK2 inhibitor GRN-300 sensitizes ovarian cancer cells to ICB and enhances T-cell cytotoxicity.
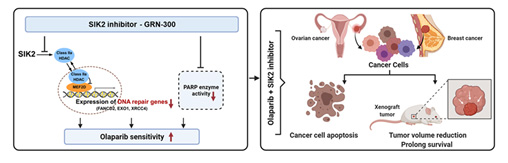
Figure 1. The combination of PARP inhibitors and SIK2 inhibitors provides a therapeutic strategy to enhance PARP inhibitor sensitivity for ovarian cancer.
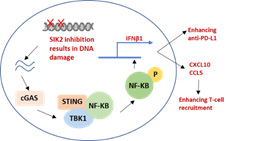
Figure 2. DNA damage through SIK2 inhibition activates the cGAS/STING pathway, leading to increase T-cell recruitment and anti-PD-L1 activity.
Project 2: Next-Generation Engineered NK Cell Immunotherapy for Ovarian Cancer
Project Co-Leaders:
Katy Rezvani, MD, PhD (Basic)
Rafet Basar, MD (Basic)
Amir Jazaeri, MD (Clinical)
Ovarian cancer is the leading cause of gynecologic cancer deaths in the US posing a critical need for new therapies. Chimeric antigen receptor (CAR) T-cell therapy has led to a paradigm shift in some hematologic cancers, but efficacy in solid tumors remains limited, partly due to the lack of highly specific targets and immunosuppression in the tumor microenvironment (TME). Moreover, the time and high cost of manufacturing autologous cell products, and the toxicity challenges related to CAR-T cells call for novel products that are universal, safe, and potent. CAR-natural killer (NK) cells have emerged as a promising and safe allogeneic cellular immunotherapy for cancer. In a first-in-human study, our group showed the safety and efficacy of cord blood (CB)-derived CD19-targeting CAR-NK cells in B-lymphoid malignancies. This proposal aims to build on this platform to develop the next-generation NK-cell therapies for ovarian cancer by enhancing NK-cell potency and persistence through optimal co-stimulatory signaling, cytokine armoring and checkpoint inhibition. We have identified TROP2 as a promising therapeutic target in ovarian cancer and developed a novel strategy to target TROP2 by genetically modifying CB-NK cells with a retroviral vector encoding: (i) TROP2-targeting hRS7 scFv; (ii) an optimized co-stimulatory domain; (iii) IL-15 to support their survival and proliferation; and (iv) inducible caspase-9 as a safety switch (iC9/TROP2CAR/IL-15). Our preliminary data show the efficacy and safety of this approach in vitro and in vivo and support its translation to the clinic. In addition, we have developed a robust strategy to cryopreserve CAR-NK cells, allowing for the generation of a biobank of off-the-shelf engineered NK cells that could be thawed and infused at bedside, thus reducing cost and increasing accessibility. Finally, we have devised a novel strategy to target an immune checkpoint to modulate the metabolic fitness and potency of CAR-NK cells in the acidic TME. We hypothesize that targeting TROP2 with iC9/TROP2CAR/IL-15 NK cells will greatly improve outcomes for platinum-resistant ovarian cancer and that by targeting an NK-cell metabolic checkpoint we can further enhance the potency of NK cells.
Specific Aims:
Aim 1: we will conduct a Phase I/II clinical trial to test the safety and efficacy of intraperitoneally delivered iC9/TROP2CAR/IL-15 NK cells in patients with TROP2+ platinum-resistant ovarian cancer (Protocol 2022-0687; Figure 1).
Aim 2: we will apply innovative single-cell proteomic and transcriptomic studies to comprehensively characterize the fate of adoptively transferred CAR-NK cells and key mechanisms of efficacy and resistance.
Aim 3: we will perform mechanistic studies to elucidate how deletion of a metabolic checkpoint enhances the fitness of CAR-NK cells and pre-IND studies for clinical translation.
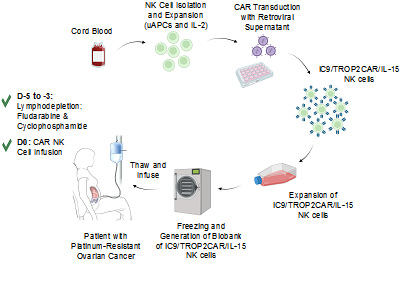
Figure 1. Schematic diagram for the process of generation and biobanking of GMP-grade off-the-shelf iC9/TROP2CAR/IL-15 NK cells.
Project 3: Targeting EGFL6 in Ovarian Cancer
Project Co-Leaders:
Anil Sood, MD (Basic)
Shannon Westin, MD (Clinical)
Timothy Yap, MD, PhD (Clinical)
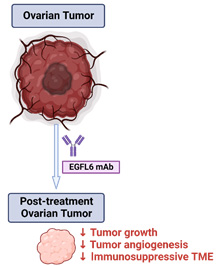
High-grade serous ovarian cancer (HGSC) is the most common and aggressive type of epithelial ovarian cancer. It is well documented that aberrant angiogenesis occurs in the tumor microenvironment (TME) and angiogenesis inhibitors are important for cancer therapy. However, the clinical benefit of bevacizumab (vascular endothelial growth factor (VEGF) targeted antibody) has been limited by rapid emergence of resistance. Moreover, therapies targeting the VEGF signaling pathway can also result in adverse events and interfere with wound healing. Thus, new targets and approaches aimed at the TME for improving therapeutic outcomes are needed. We identified epidermal growth factor (EGF)-like domain multiple 6 (EGFL6) as the most differentially expressed gene in tumor endothelial cells compared to endothelial cells from normal ovarian tissues and healing wounds. Our preliminary data suggest that high EGFL6 expression in tumors is associated with an immune suppressive TME with high M2 macrophage infiltration. To develop a therapeutic approach for blocking EGFL6, we developed and tested a large number of candidate antibodies; the final candidates have been humanized. Our in vivo results indicated that these antibodies had robust anti-tumor effects and reduced angiogenesis in ovarian cancer models. Based on our compelling preliminary data, we hypothesize that EGFL6 promotes aberrant angiogenesis, and immune suppression, resulting in ovarian cancer growth and progression.Blocking EGFL6 with a monoclonal antibody provides a novel and effective approach for treatment of ovarian cancer.
Specific Aims:
Aims 1: To delineate the molecular regulation of EGFL6 and identify sources of EGFL6 in the tumor microenvironment.
Aim 2: To investigate the biological effects of anti-EGFL6 monoclonal antibody as monotherapy or in combination with chemotherapy, anti-VEGF antibody, or immune checkpoint inhibitor.
Aim 3: To determine the safety and tolerability of an anti-EGFL6 antibody in a first-in-human, first-in-class phase I clinical trial in patients with recurrent ovarian cancer. Collectively, the work proposed in this project will provide scientific rationale for developing new anti-EGFL6 based therapies. The proposed studies will provide fundamental mechanistic insights into the role of EGFL6 in regulating immune responses in the TME.
Project 4: Targeting p300 to Overcome PARP inhibitor resistance induced by acidic tumor microenvironment
Project Co-Leaders:
Rugang Zhang, PhD (Basic)
Shannon Westin, MD (Clinical)
The overall goal of this proposal is to develop a novel strategy to improve efficacy and/or overcome resistance to PARP inhibitors induced by acidic tumor microenvironment by targeting epigenetic regulator p300. PARP inhibitors such as olaparib are FDA-approved for the maintenance and treatment of epithelial ovarian cancer (EOC) patients with impaired homologous recombination (HR) pathways, most notably with BRCA1/2 mutations (also known as homologous recombination deficiency or HRD). However, resistance to PARP inhibitors remains a major unmet clinical need. This is a hypothesis-driven translational study, and the findings will be pivotal for evaluating whether IACS16559, a p300 inhibitor developed by the MD Anderson Cancer Center, in combination with olaparib represents an effective approach to improve efficacy and/or overcome resistance to PARP inhibitors. Substantial evidence shows that tumors exhibit a lower extracellular pH compared to normal tissues. Our preliminary data show that acidic extracellular pH causes resistance to PARP inhibitors such as olaparib in HRD EOC cells. Our CRISPR screen revealed p300, an epigenetic transcription activator, as a top hit whose inhibition sensitizes cells to olaparib in acidic pH. Mechanistically, our preliminary data indicated that in a p300-dependent manner, acidic pH suppresses the non-homologous end joining (NHEJ) DNA double-strand break (DSB) repair pathway and reduces PARP trapping. Our central hypothesis is that targeting p300 using clinically applicable small molecule inhibitor IACS16559 is a promising therapeutic strategy to improve the efficacy and/or overcome the resistance to PARP inhibitors caused by acidic tumor microenvironment in HRD EOCs.
Specific Aims:
Aim 1: Will investigate a combined therapeutic strategy of targeting p300 and olaparib in HRD EOC cells and patient-derived xenografts.
Aim 2: Will explore the combination of p300 inhibitor IACS16559 and olaparib in patients with HRD non-mucinous EOC in a Phase 1 clinical trial.
Aim 3: Will identify biomarkers that correlate with response to IACS16559 and olaparib combination in HRD EOCs. The proposed studies are of high impact because they will develop novel therapeutic strategies to improve the efficacy and/or overcome the resistance of PARP inhibitors by targeting p300 with IACS16559 in combination with olaparib, a major challenge in the clinical management of ovarian cancer.
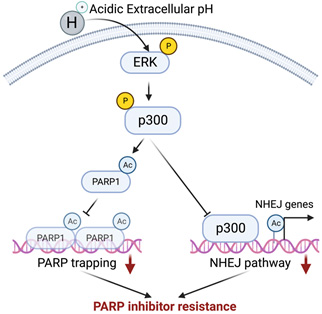
A working model by which acidic extracellular pH contributes to PARP inhibitor resistance.
Administrative Core
Core Co-Directors:
Anil Sood, MD
Robert Bast, Jr., MD
The overall goal of the MD Anderson Cancer Center SPORE in Ovarian Cancer is to translate high-quality research into improved clinical outcomes for women with ovarian cancer. To ensure the success of the SPORE, the Administrative Core will provide effective management and administration of all activities relating to each project, core, career enhancement (CEP) and developmental program (DRP) in the SPORE. The Administrative Core will communicate and disseminate information between our SPORE investigators and facilitate information sharing and collaborations with other SPOREs and external collaborators. The Core will provide a platform for integrating diverse scientific disciplines into a unified multidisciplinary approach for achieving excellence in translational ovarian cancer research. The Administrative Core will monitor timelines and ensure both basic science and clinical trial aims are met for all SPORE projects. Co-Core Directors and MPIs, Drs. Anil Sood and Robert Bast, along with Ovarian SPORE Administrator, Ms. Ersulan Hampton, will direct the objectives of the Administrative Core.
Specific Aims:
Aim 1: Direct the planning and evaluation to ensure the overall scientific quality and strategic direction of the SPORE.
Aim 2: Manage all fiscal and budgetary issues across all Projects, Cores and DRP/CEP.
Aim 3: monitor the data sharing compliance of the SPORE to adhere to all general governmental and specific NCI regulations and requirements.
Aim 4: Coordinate quality assurance activities, including data quality controls with support from the Bioinformatics and Biostatistics Core and the Pathology Core.
Aim 5: Serve as the communication hub for the SPORE to provide up-to-date operating information to investigators.
Aim 6: Communicate with the NCI Project Officer and staff to make certain all necessary reporting requirements are met, including PMCID policy compliance.
Aim 7: Coordinate the annual process for selection of DRP/CEP recipients, and establish policies and procedures that result in the successful recruitment of women and underrepresented minorities to these programs and ensure awardees are supported with outstanding mentorship.
Aim 8: Ensure the SPORE is integrated in administrative organization of the institution.
Aim 9: initiate and coordinate inter-SPORE and inter-institutional interactions and seminars.
Aim 10: Facilitate resolution of scientific disputes.
Aim 11: Organize and maintain patient advocate involvement in the SPORE.
Bioinformatics and Biostatistics Core
Core Co-Directors:
Ying Yuan, PhD
Han Liang, PhD
Jing Ning, PhD
The primary goal of the Bioinformatics and Biostatistics Core is to provide centralized biostatistics, bioinformatics, and database support for all SPORE projects and cores, ranging from basic science studies to animal studies to genomics studies to clinical trials. The Core will serve multiple needs for the planning and conduct of the SPORE's translational research. Based on a strong track record of providing biostatistical support for translational research, the Core will be a comprehensive, multilateral resource for designing clinical and basic science experiments, performing statistical analyses, developing innovative statistical methodology, and publishing the research results generated from the SPORE. The Core will confer regularly with project investigators to discuss the design and conduct of research projects, evaluate results of analyses, discuss potential new research initiatives and directions within the SPORE, and promote the publication of findings.
Specific Aims:
Aim 1: Provide statistics and data analyses required for the Projects and Cores to achieve their specific aims.
Aim 2: Provide bioinformatics expertise required for analyses and interpretation of high-throughput assay data.
Aim 3: Assist with design and implementation of new trials and studies arising from SPORE research.
Aim 4: As necessary, to develop and adapt innovative statistical and bioinformatics methods pertinent to ovarian cancer studies.
Pathology Core
Core Director
Jinsong Liu, MD, PhD
The individual research projects comprising this Ovarian Cancer SPORE application require the procurement, processing, and analysis of histopathological material from patients with ovarian cancer and benign ovarian diseases. The research projects have needs for frozen and formalin-fixed, paraffin-embedded samples of tumor and normal tissue. The research projects also need patient-derived xenograft models and organoids to test the effects of novel drugs and define the mechanisms of drug resistance. The proposed Pathology Core augments the already established MD Anderson Cancer Center Gynecological Tumor Bank and the P30-sponsored MD Anderson Cancer Center Centralized Tissue Repository with supporting database and intranet access. The Core provides for tissue acquisition by experienced gynecological pathologists to assure high-quality tissues for the investigators participating in this SPORE as well as investigators of other SPOREs.
The goal of the Pathology Core is to provide frozen tissue, paraffin-embedded tissue, histopathological expertise, tissue arrays, patient-derived xenografts, and organoids related to the specific needs of the research projects comprising this SPORE proposal.
Specific Aims
Aim 1 is to maintain a frozen and paraffin-embedded tissue repository of ovarian cancers, normal ovaries, and benign ovarian processes (such as cysts and endometriosis).
Aim 2 is to provide pathological review for all clinical specimens utilized in the SPORE projects and to provide histopathological and immunohistochemical technical services as necessary.
Aim 3 is to create tissue microarrays, patient-derived xenografts (PDXs), and organoid models and maintain a relational SPORE Database. This SPORE Database will provide for a virtual tissue repository that can be shared electronically with all SPORE investigators.
Developmental Research Program
Program Director:
Robert Bast, Jr., MD
The Developmental Research Program (DRP) is designed to take advantage of the rich diversity of basic, translational, and clinical expertise at MD Anderson and in the Texas Medical Center and other participating institutions to enhance innovative research focused on early diagnosis, treatment, and prevention of ovarian cancer. The Ovarian SPORE will fund two DRP awards, each of $50,000 annually for up to two years. The purpose of the MD Anderson Ovarian SPORE DRP is to encourage and develop research projects that will lead to clinically testable hypotheses that will reduce ovarian cancer incidence and mortality or improve survival and quality of life for women with this disease.
The DRP component of the SPORE serves as a mechanism to increase the number of scientists and clinical investigators who are committed to translational research in ovarian cancer. The central premise of the DRP is that support of developmental research projects will enable exploration of novel and meritorious approaches for early detection and treatment of ovarian cancer. The DRP projects will be carried out in a supportive environment of basic, translational, and clinical research that is created by SPORE investigators.
Specific Aims:
Aim 1: To identify and facilitate innovative research that has clear translational potential to address important problems in early detection, prevention, and management of ovarian cancer.
Aim 2: To provide mechanisms for enabling collaborative research that bridges basic, translational, and clinical arenas to advance research on ovarian cancer.
Aim 3: To attract a diverse group of investigators from allied fields who will apply their specific areas of expertise to ovarian cancer research.
Aim 4: To identify candidate projects that could mature into full SPORE projects in the future.
Career Enhancement Program
Program Director:
Robert Bast, Jr., MD
The central goal of the Career Enhancement Program (CEP) is to use the resources at MD Anderson Cancer Center to train exceptional young investigators who will reduce the morbidity and mortality from ovarian cancer by making advances in the early detection, prevention, and treatment of this disease. To achieve this goal, the MD Anderson Ovarian SPORE will fund two CEP awards, each of $50,000 annually for up to two years. The intent of each CEP award is to prepare the selected scientists to become international leaders in academic research relevant to ovarian cancer. We will achieve this through focused recruitment of a diverse cadre of promising young investigators. We will work in partnership with awardees to generate individualized development plan and provide them with outstanding clinical and a laboratory mentors and a mentoring committee, formal course work, coaching in grant and paper writing, leadership training, attendance at national meetings, networking with ovarian cancer scholars and completing and publishing a translational ovarian cancer research program.
The positive impact of the CEP is that support of career enhancement projects will enable exploration of novel and meritorious approaches for early detection and treatment of ovarian cancer. Furthermore, CEP projects will be carried out in a supportive environment of basic, translational, and clinical research created by the experienced SPORE investigators.
The objective of the CEP is to build a strong, diverse ovarian cancer CEP team that will mentor and foster the career development of the CEP awardees in the field of ovarian cancer research. The SPORE leadership will support these awardees’ projects both with scientific mentorship as well as with research funding.
Specific Aims:
Aim 1: To recruit and train a diverse group of outstanding investigators to develop innovative translational approaches for improving ovarian cancer diagnosis, prevention, and treatment.
Aim 2: To educate awardees in the current principles of ovarian cancer biology and clinical care with an emphasis on translational science.
Aim 3: To integrate the CEP and other programs within and outside the Ovarian Cancer SPORE.







