SPORE in Cervical Cancer
Johns Hopkins University
Principal Investigators:

T.-C. Wu, MD, PhD

Warner Huh, PhD
- Principal Investigators Contact Information
- Overview
- Project 1: Thermostable RG-1 VLPs, A Candidate Broadly Protective HPV Vaccine for the Prevention of Cervical Cancer Globally
- Project 2: Jet vaccination with pBI-11 DNA to treat HPV16/18+ ASC-US/LSIL
- Project 3: Electroporation delivery of pNGVL4aCRTE6E7L2 DNA for treatment of HPV16+ CIN2/3 patients
- Project 4: A Phase I safety & immunogenicity study of an HPV16-specific therapeutic protein, TA-CIN, administered intratumorally with chemoradiation in patients with cervical cancer stratified by HPV16 infection
- Administrative/Communication Core
- Biostatistics and Bioinformatics Core
- Tissue/Pathology/Immunology Core
- Developmental Research Program
- Career Enhancement Program
- Institutional SPORE Website
Principal Investigators Contact Information
T.-C. Wu, MD, PhD
Professor of Pathology
Director, Gynecologic Pathology Division
Director, Cervical Cancer Research Lab
1550 Orleans St, CRB II Room 309,
Baltimore, Maryland, 21231
(410) 614-3899
Warner Huh, PhD
Chair, Department of Obstetrics/Gynecology Medical Director
Endowed Chair in Obstetrics/Gynecology Professor,
Department of Obstetrics/Gynecology
University of Alabama at Birmingham
1700 6th Avenue South, WIC 10360,
Birmingham, Alabama 35233
(205) 934-1555
Overview
Every year, 604,000 women are diagnosed with cervical cancer, and 342,000 die from it worldwide. Over the next five years, our cervical cancer SPORE aims to enhance outreach and protection for the next generation of women against oncogenic HPV infections. We also aim to pioneer new vaccines to improve treatment outcomes for patients with persistent HPV infections, HPV-associated precancerous conditions, and cervical cancer itself. Vaccination is recognized as the most effective and economically viable public health measure available.
Our SPORE includes four distinct projects, building on the successes of prophylactic HPV vaccines in primary cervical cancer prevention and leveraging advancements in cellular immunology. Efforts will focus on elucidating mechanisms for spontaneous viral clearance and assessing HIV's impact on these processes.
The primary objective of the Cervical Cancer SPORE Program over the next five years is to develop innovative vaccines that enhance treatment outcomes for individuals with HPV-related cervical cancer and its precursors. Simultaneously, we aim to expand outreach and protect the next generation of women from oncogenic HPV infections and their complications.
The program outlines three overarching goals: Firstly, PRIMARY PREVENTION to reduce global cervical cancer incidence by improving access to a low-cost, thermally stable RG1-VLP formulation. This formulation, in a single dose, effectively prevents infection by all oncogenic HPV types (Project 1). Secondly, SECONDARY PREVENTION to eliminate persistent HPV infections (Project 2) and associated precancerous lesions (Project 3) using innovative therapeutic HPV vaccines. Thirdly, the program improves CANCER TREATMENT outcomes for advanced cervical cancer by integrating an innovative therapeutic HPV vaccination post-chemoradiation to target minimal residual disease (Project 4).
To support these efforts, the program includes essential cores: Administrative/Communication (Core A), Biostatistics/Bioinformatics (Core B), and Tissue/Pathology/Immunology (Core C). Additionally, it fosters innovation and renewal through a Developmental Research Program (DRP) and a Career Enhancement Program (CEP). Our interdisciplinary team collaborates closely across molecular and cellular biology, virology, immunology, pathology, medical and surgical oncology, and cervical cancer epidemiology. Together, they aim to rapidly translate laboratory breakthroughs into novel, cost-effective preventive and therapeutic strategies. Ultimately, the program strives to reduce global cervical cancer incidence, diminish morbidity, and lower mortality rates worldwide.
Project 1: Thermostable RG-1 VLPs, A Candidate Broadly Protective HPV Vaccine for the Prevention of Cervical Cancer Globally
Project Co-Leaders:
Richard Roden, PhD (JHU) (Basic Science Co-Leader)
Richard Kimbauer, MD (MedUni Vienna) (Clinical Co-Leader)
Robert Garcea, MD (UC Boulder) (Clinical Co-Leader)
Theodore Randolph, PhD (UC Boulder) (Basic Science Co-Leader)
HPV causes 10% of all women’s cancers globally, including 99% of cervical cancers. Rates vary widely by region and economic status, with over 85% of cases in developing countries. Many poorer populations lack access to HPV vaccination and cervical screening. There are 13 high-risk HPV types and 11 possibly carcinogenic types. Current vaccines target HPV16 and HPV18, with Gardasil®9 also addressing the next five common types in cervical cancer. However, none cover all cancer-associated HPVs, necessitating continued screening even for vaccinated individuals and increasing medical costs. Challenges include the cold-chain requirement and multiple doses for vaccines, which hinder delivery in developing nations.
Our goal is an affordable HPV vaccine offering broad protection against all cancer-associated HPV types, requiring only one administration and stable at ambient temperatures. We pursue this with two innovations: first, displaying the RG1 protective epitope on HPV16 L1 virus-like particles (VLPs), creating a single antigen, broadly protective vaccine (RG1-VLP); second, formulating RG1-VLP through freeze-drying in an organic matrix with Al2O3 atomic layer deposition for heat stability, protection during delivery, and sustained antigen release suitable for a single-dose regimen.
Specific Aims:
Aim 1: To perform a dose escalation phase I trial of the safety and immunogenicity of RG1-VLP absorbed to alum in healthy female volunteers.
Aim 2: To analyze the levels of protection against intravaginal HPV challenge conferred by passive transfer of serum of patients vaccinated with either RG1-VLP or Gardasil®9 in mice with fully humanized Fc receptors or none.
Aim 1: Develop an ALD coated RG1-VLP vaccine, study its in vitro temperature stability, and in murine models test its one dose immunogenicity and protective efficacy and breadth of coverage in comparison to standard regimen Gardasil®9 vaccination.
Project 2: Jet vaccination with pBI-11 DNA to treat HPV16/18+ ASC-US/LSIL
Project Co-Leaders:
Chien-Fu Hung, PhD (JHU) (Basic Science Co-Leader)
Kimberly Levinson, MD (JHU) (Clinical Co-Leader)
Rebecca Arend, MD (UAB) (Clinical Co-Leader)
HPV16, the predominant oncogenic genotype, causes over half of global cervical cancer cases, with HPV18 contributing another 20%. Current preventive HPV vaccines do not treat HPV16/18 infections identified through cervical screening. Nearly half of persistent HPV16 infections and a quarter of HPV18 progress to high-grade cervical intraepithelial neoplasia (CIN2/3), with risk increasing with age. Persistent HPV16/18 infections pose transmission risks and psychological stress due to their oncogenic potential.
There is an urgent need for a therapeutic intervention to eliminate HPV16/18 infections before cancer develops. The goal is to develop a regimen that safely clears these infections, intercepting progression to cervical cancer. A DNA vaccine, pBI-11, encoding HPV16/18 E6 and E7 proteins linked to heat shock protein 70 (HSP70), enhances antigen-specific CD8+ T cell immunity and efficacy in mice. However, DNA vaccines alone have limited potency in humans due to delivery challenges.
Needle-free delivery of ZyCov-D, a naked DNA vaccine, demonstrated safe and effective delivery for SARS-CoV-2 prevention in India, highlighting its potential for potent vaccine delivery in HPV-associated cancers. This intervention aims not only to prevent cervical cancer precursor lesions and cervical cancer but also other HPV-related anogenital and oropharyngeal cancers.
In summary, developing an effective therapeutic approach to clear persistent HPV16/18 infections is critical to preventing cervical cancer progression and associated morbidities. Leveraging advanced delivery methods like ZyCov-D offers promise in enhancing the potency of DNA vaccines for broader HPV-related cancer prevention.
Specific Aims:
Aim 1: To assess the safety, tolerability and feasibility of repeat intradermal or intramuscular vaccination with pBI-11 DNA via a needle-free jet device in women with HPV16 and/or HPV18+ <CIN2 cervical disease.
Aim 2: To determine the immunogenicity of intradermal or intramuscular jet administration of pBI-11 DNA to women with HPV16/18+ <CIN2 cervical disease.
Aim 3: To examine virologic, cytologic, and pathologic changes at the cervix upon repeat intradermal or intramuscular needle-free jet administration of pBI-11 DNA in women with HPV16 and/or HPV18+ <CIN2 cervical disease.
Project 3: A Phase I Open Label, Dose Escalation Clinical Trial Assessing the Safety, Tolerability, and Feasibility of pNGVL4aCRTE6E7L2 HPV DNA Vaccine Administration Via Intramuscular TriGridTM Electroporation
Project Co-Leaders:
Kimberly Levinson, MD (JHU) (Clinical Co-Leader)
Richard Roden, PhD (JHU) (Basic Science Co-Leader)
Warner Huh, MD (UAB) (Clinical Co-Leader)
Surgical or ablative treatment for cervical cancer precursors, such as high-grade squamous intraepithelial lesion (HSIL) or cervical intraepithelial neoplasia (CIN2/3), has reduced disease incidence but is linked to higher rates of infectious complications, cervical incompetence leading to premature deliveries, failure to clear HPV, and disease recurrence. Persistent HPV infections can also lead to pre-invasive and invasive diseases in the vagina, vulva, and anus.
Our objective is to evaluate the safety and toxicity of the CRTE6E7L2 DNA vaccine, administered intramuscularly via the TriGrid Electroporation Device, in HIV-positive and HIV-negative patients who have HPV16-associated HSIL lesions in the cervix, vagina, and/or vulva. We will assess virologic and disease outcomes. HPV16 is prevalent in cervical cancer and dominates in other anogenital and head and neck cancers (>85%).
The CRTE6E7L2 vaccine combines the pNGVL4a DNA vector encoding heat shock protein calreticulin (CRT) fused genetically with HPV16 E6 and E7 proteins, essential in HPV malignancies, and the L2 capsid protein, providing broad antigenic protection. CRT fusion enhances DNA vaccine potency in generating HPV-specific CD8+ T cell responses. CRTE6E7L2 induces L2-specific neutralizing antibodies and protects against experimental vaginal challenge, making it promising for HIV-positive and transplant patients. In vivo electroporation significantly improves DNA vaccine effectiveness compared to traditional intramuscular injection, offering robust HPV-specific CD8+ T cell responses. Early clinical responses in both HIV-positive and negative patients have been highly promising.
Specific Aims:
Aim 1: Evaluate the safety and toxicity of CRTE6E7L2 administered via electroporation in HIV- and HIV+ patients with HPV16+ high-grade CIN/VaIN/VIN, including HIV- patients on the kidney transplant waitlist
Aim 2: Characterize the HPV16 E6/E7/L2-specific cell-mediated responses to vaccination of each cohort.
Aim 3: Characterize the HPV16 E6/E7/L2-specific humoral immune responses to vaccination of each cohort
Aim 4: Determine the HPV and histopathological responses in the lesion and its microenvironment to vaccination in each cohort.
Project 4: Therapeutic HPV vaccines (PVX7) for Advanced Cervical Cancer
Project Co-Leaders:
T.-C. Wu, MD PhD (JHU) (Basic Science Co-Leader)
Stephanie Gaillard, MD PhD (JHU) (Clinical Co-Leader)
Charles Leath III, MD (UAB) (Clinical Co-Leader)
The challenge in managing advanced cervical cancer lies in identifying patients with residual disease at risk of recurrence and metastasis, and preventing such occurrences with novel immunotherapies. This project aims to achieve two goals: Firstly, to conduct a pilot study on a new therapeutic HPV vaccine's safety and tolerance in women who completed standard cervical cancer therapy but may still harbor minimal residual disease. Secondly, to investigate whether cell-free HPV DNA (cfHPVDNA) in plasma can serve as a biomarker for HPV+ cancer persistence.
Our adjuvant immunotherapy targets HPV16 and HPV18 E6 and E7 proteins, responsible for over 70% of cervical cancer cases. This involves priming with pBI-11 DNA encoding these proteins fused with heat shock protein 70 (HSP70), enhancing antigen presentation. Previous studies using similar approaches showed promising results in HPV16+ CIN2/3 patients.
We hypothesize that after initial pBI-11 DNA vaccination, boosting with TA-HPV vaccinia will induce stronger HPV-specific CD8+ T cell responses compared to intramuscular boost. This approach aims to control residual cervical cancer post-standard therapy (surgery/chemoradiation).
Ultra-sensitive cfHPVDNA measurement in plasma shows potential as a biomarker for monitoring cervical cancer burden and identifying high-risk individuals for recurrence. Adapting an FDA-approved PCR-based test for cfHPVDNA measurement globally is feasible based on preliminary data.
Specific Aims:
Aim 1: To assess the safety and feasibility of the pBI-11 DNA prime followed by TA-HPV boost (PVX7), wherein TA-HPV is administered via either IM injection or SS, in patients with advanced cervical cancer who have completed primary therapy and have no clinical evidence of disease.
Aim 2: To evaluate the systemic HPV16/18 E6/E7-specific cellular immune responses to PVX7 immunotherapy regimens with TA-HPV administered via IM or SS in patients with advanced cervical cancer who have completed primary therapy.
Aim 3: To assess cell-free HPV16/18 DNA load in plasma pre- and post-PVX7 immunotherapy using different HPV genome detection methods, and characterize progression- free and overall survival for patients with advanced cervical cancer.
Administrative/Communication Core
Core Directors:
T.-C. Wu, MD PhD (JHU)
Warner Huh, MD (UAB)
The Administrative/Communication Core is responsible for managing and overseeing the SPORE, disseminating information within the SPORE, and external interactions.
The objectives include:
- Provide administrative oversight to all activities of the SPORE, including Projects, Cores and Programs.
- Oversee all communication and consultation with the NCI personnel in the preparation of all required reports and publications.
- Enhance interaction and communications between JHU, UAB, and UCB by convening all meetings.
- Assume responsibility for maintenance of fiscal and budgetary functions.
- Establish and monitor policies for the recruitment of individuals from disadvantaged groups in all clinical studies and as SPORE investigators.
- Ensure protection of human subjects from research risks with implementation of a data safety and monitoring plan where appropriate.
- Assume responsibility for all issues related to technology transfer and management of intellectual property rights under the requirements of the Bayh-Dole Act NIH funding agreements.
- Ensure that the SPORE interactions with commercial entities uphold the principles of academic freedom, including the ability of SPORE investigators to collaborate freely and to send and receive biomedical research materials without undue restriction to other scientific investigators
Biostatistics and Bioinformatics Core
Core Directors:
Sejong Bae, PhD (UAB)
Hao Wang, PhD (JHU)
Heba Mostafa, MD PhD (JHU)
The Biostatistics and Bioinformatics Core will provide centralized statistical services and molecular assay validation support for the research program of the SPORE for cervical cancer. The primary objective of this core is to provide aid and direction in experimental design, assay methodology, quantitative data analysis and interpretation through consultation and collaboration. The specific aims are:
- Provide biostatistical and bioinformatical consultation and support by assisting in experimental design and data collection, visualization, analysis, quantitative modeling, interpretation, publication and sharing for all SPORE projects.
- Provide biostatistical and bioinformatical support for the Tissue/Pathology/Immunology Core C, the Developmental Research Program (DRP) awardees, and the Career Enhancement Program (CEP) awardees.
- Advice and support the development of centralized facilities and protocols for data storage, management, and dissemination.
- Support HPV and other molecular testing development and validation that complies with CLIA regulations for the clinical testing proposed in the SPORE projects and provide biomarker assay research methodology and experiment design support for the Tissue/Pathology/Immunology Core C, the DRP awardees, and the CEP awardees.
Tissue/Pathology/Immunology Core
Core Directors:
Russell Vang, MD (JHU)
Raphael Viscidi, MD (JHU)
Andrea Kahn, MD (UAB)
Rebecca Arend, MD (UAB)
The acquisition and preservation of well-annotated, high-quality human tissues and biologic fluids, and the in situ analysis of the immune response therein, is pivotal to the success of all four HPV vaccine-based SPORE projects.
Specifically, Tissue/Pathology/Immunology Core aims to:
- Collect, process, store, and ship tissues and biologic fluids for translational research without compromising sample integrity, traceability, or patient care
- Provide well-characterized tumors, precursor lesions and normal tissues with respect to site of origin, World Health Organization histologic subtype, differentiation, and fraction of neoplastic and stromal tissues with matching PBMC or serum samples as required
- Support the generation of tissue microarrays with a wide range of biologic potential (e.g. normal, dysplasia, carcinoma) and/or relevant clinical parameters (e.g. before and after vaccination) and laser-capture microdissection as needed
- Provide expert advice, execution and interpretation of multi-parameter immunohistochemical studies including digital microscopic photography and image processing
- Provide pathological interpretation of stained tissue and cytologic samples
- Organize blinded sample sets to ship to sites that include appropriate controls to ensure data integrity, reproducibility and comparability.
Developmental Research Program
Program Directors:
T.-C. Wu, MD PhD (JHU)
Drew Pardoll, MD PhD (JHU)
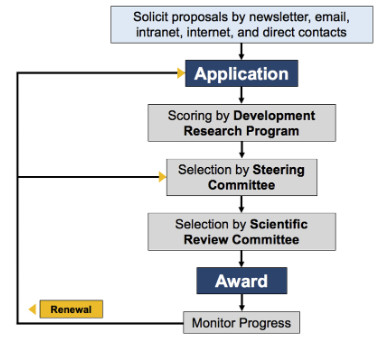
The Developmental Research Program is an important component of the SPORE and critical to the long-term fight against cervical cancer. It provides an avenue for soliciting new research ideas and for developing innovative high-risk, but high-impact projects to stimulate cervical cancer research in the context of the SPORE. Pilot studies provide investigators with the resources to conduct translational research consistent with the SPORE’s objectives. This program will encourage participation from a broad range of investigators at Johns Hopkins and University of Alabama Birmingham (UAB) by providing support for pilot projects with the potential to develop into more fully developed translational projects. It will also encourage and facilitate the development of new research directions, methodologies, and collaborations. In addition, the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins also provides $75,000 annually as match funds for the Developmental Research Program. UAB will also contribute $40,000 annually to be used in the Career Development or Developmental Research program.
The Developmental Research Program Process
Career Enhancement Program
Program Directors:
T.-C. Wu, MD PhD (JHU)
Donald Buchsbaum, PhD (UAB)
Clayton Yates, PhD (JHU)
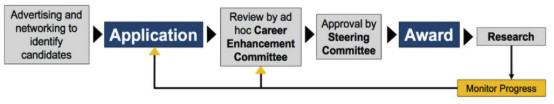
The Career Development Program will allow the Cervical Cancer SPORE to attract and stimulate young investigators to carry out translational research related to cervical cancer. Candidates will submit an application and be evaluated through a careful selection process. Recipients of the award will be reviewed annually, and investigators are required to submit an annual progress report subject to the aforementioned review process.
In order to increase the opportunity for investigators to participate from underrepresented racial and ethnic groups, we will also take advantage of the respective NCI funded Minority Institution/Comprehensive Cancer Center Partnerships at our respective institutions. Thus, the CEP will also be available to junior investigators at Historically Black Colleges and Universities (HBCU) including, Morehouse School of Medicine and Tuskegee University as part of the UAB Partnership and with Howard University, a part of the JHU Partnership.
Mechanisms for identifying, selecting, and funding qualified candidates